Oral intake of Kluyveromyces marxianus B0399 plus lactobacillus rhamnosus CECT 30579 to mitigate symptoms in COVID-19 patients: A randomized open label clinical trial
et al., Medicine in Microecology, doi:10.1016/j.medmic.2022.100061, NCT04390477, Aug 2022
Probiotics for COVID-19
20th treatment shown to reduce risk in
March 2021, now with p = 0.00000044 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
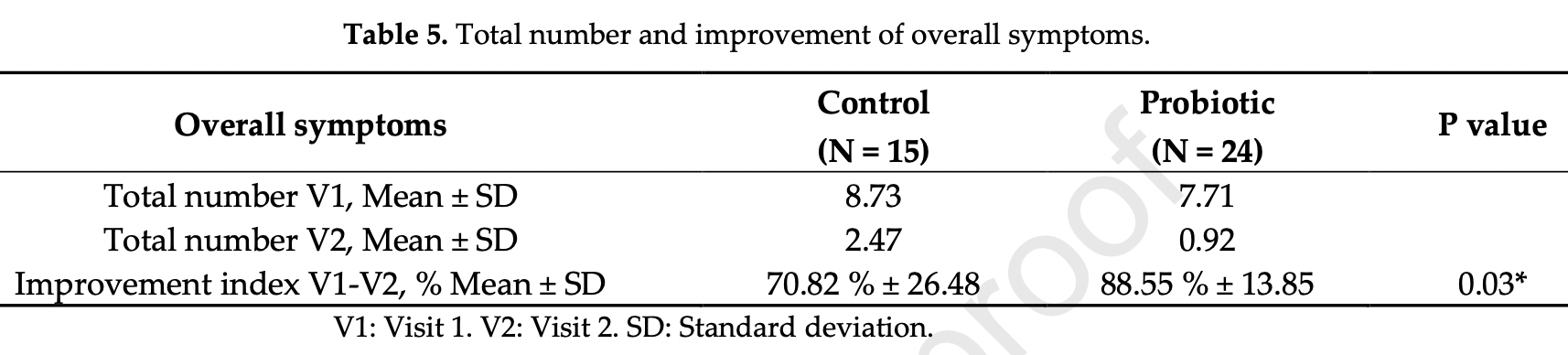
RCT with 24 probiotics and 15 control patients in Spain, showing lower overall symptoms and lower digestive symptoms with treatment. Kluyveromyces marxianus B0399 plus lactobacillus rhamnosus CECT 30579.
Probiotic efficacy depends on the specific strains used. Specific microbes may decrease or increase COVID-19 risk1.
|
risk of no recovery, 32.7% lower, RR 0.67, p = 0.08, treatment 14 of 24 (58.3%), control 13 of 15 (86.7%), NNT 3.5, day 30.
|
|
risk of no recovery, 53.1% lower, RR 0.47, p = 0.10, treatment 6 of 24 (25.0%), control 8 of 15 (53.3%), NNT 3.5, digestive symptoms, day 30.
|
|
relative recovery, 20.0% better, RR 0.80, p = 0.03, treatment 24, control 15, relative symptom improvement, day 30.
|
|
relative recovery, 26.1% better, RR 0.74, p = 0.06, treatment 24, control 15, relative improvement for digestive symptoms, day 30.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Navarro-López et al., 24 Aug 2022, Randomized Controlled Trial, Spain, peer-reviewed, 13 authors, study period December 2020 - February 2021, trial NCT04390477 (history).
Contact: vnavarro@ucam.edu (corresponding author), pedro.sanchez@bioithas.com, juan.aguera@bioithas.com, eva.nunez@bioithas.com, beatriz.ruzafa@bioithas.com, laura.navarro@bioithas.com.
Oral intake of Kluyveromyces marxianus B0399 plus Lactobacillus rhamnosus CECT 30579 to mitigate symptoms in COVID-19 patients: A randomized open label clinical trial
Medicine in Microecology, doi:10.1016/j.medmic.2022.100061
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations The journal requires that the corresponding author, signs on behalf of all authors, a declaration of conflicting interests. If you have nothing to declare in any of these categories then this should be stated.
Medicine in Microecology
J o u r n a l P r e -p r o o f No conflict of interest the author ensure that the work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Conflict of Interest A conflicting interest exists when professional judgment concerning a primary interest (such as patient' s welfare or the validity of research) may be influenced by a secondary interest (such as financial gain or personal rivalry). It may arise for the authors when they have financial interest that may influence their interpretation of their results or those of others. Examples of potential conflicts of interest include employment, consultancies , stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding. If there are no interests to declare then please state this: ' The authors declare that there are no conflicts of interest.' Please state any competing interests:
Ethical approval and informed consent (if applicable) If the work involves the use of human subjects, the author should ensure that the work described has been carried out in accordance with The Code of Ethics of the World Medical Association..
References
Becattini, Taur, Pamer, Antibiotic-Induced Changes in the Intestinal Microbiota and Disease, Trends Mol Med, doi:10.1016/j.molmed.2016.04.003
Bourgonje, Abdulle, Timens, Hillebrands, Navis et al., Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19), J Pathol, doi:10.1002/path.5471
Cardinale, Capurso, Ianiro, Gasbarrini, Arcidiacono et al., Intestinal permeability changes with bacterial translocation as key events modulating systemic host immune response to SARS-CoV-2: A working hypothesis, Dig Liver Dis, doi:10.1016/j.dld.2020.09.009
Cecchini, Zanvit, Miclavez, Nobili, Halitosis Treatment Through the Administration of Antibiotic-Resistant Probiotic Lactic Yeast Kluyveromyces marxianus fragilis B0399 (K-B0399), Biomedical Journal of Scientific and Technical Research
Cristofori, Dargenio, Dargenio, Miniello, Barone et al., Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body, Front Immunol, doi:10.3389/fimmu.2021.578386
Ezechukwu, Diya, Egoh, Abiodun, Grace et al., Lung microbiota dysbiosis and the implications of SARS-CoV-2 infection in pregnancy, Ther Adv Infect Dis, doi:10.1177/20499361211032453
Foladori, Cutrupi, Segata, Manara, Pinto et al., SARS-CoV-2 from faeces to wastewater treatment: What do we know? A review, Sci Total Environ, doi:10.1016/j.scitotenv.2020.140444
Fonseca, Heinzle, Wittmann, Gombert, The yeast Kluyveromyces marxianus and its biotechnological potential, Appl Microbiol Biotechnol, doi:10.1007/s00253-008-1458-6
Gomaa, Human gut microbiota/microbiome in health and diseases: a review, Antonie Van Leeuwenhoek, doi:10.1007/s10482-020-01474-7
Gutierrez-Castrellon, Gandara-Marti, Abreu, Nieto-Rufino, Lopez-Orduna et al., Probiotic improves symptomatic and viral clearance in Covid19 outpatients: a randomized, quadruple-blinded, placebo-controlled trial, Gut Microbes, doi:10.1080/19490976.2021.2018899
Jernberg, Lofmark, Edlund, Jansson, Long-term impacts of antibiotic exposure on the human intestinal microbiota, Microbiology (Reading, doi:10.1099/mic.0.040618-0
Kim, Guevarra, Kim, Kwon, Kim et al., Role of Probiotics in Human Gut Microbiome-Associated Diseases, J Microbiol Biotechnol, doi:10.4014/jmb.1906.06064
Kirtipal, Kumar, Dubey, Dwivedi, Gireesh Babu et al., Understanding on the possible routes for SARS CoV-2 invasion via ACE2 in the host linked with multiple organs damage, Infect Genet Evol, doi:10.1016/j.meegid.2022.105254
Labandeira-Garcia, Labandeira, Valenzuela, Pedrosa, Quijano et al., Drugs Modulating Renin-Angiotensin System in COVID-19 Treatment, Biomedicines, doi:10.3390/biomedicines10020502
Leal-Martinez, Abarca-Bernal, Garcia-Perez, Gonzalez-Tolosa, Cruz-Cazares et al., Effect of a Nutritional Support System to Increase Survival and Reduce Mortality in Patients with COVID-19 in Stage III and Comorbidities: A Blinded Randomized Controlled Clinical Trial, Int J Environ Res Public Health, doi:10.3390/ijerph19031172
Li, He, Zhu, Lu, Role of gut microbiota on intestinal barrier function in acute pancreatitis, World J Gastroenterol, doi:10.3748/wjg.v26.i18.2187
Li, Pei, Chen, Song, Zhang et al., Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (COVID-19), medRxiv, doi:10.1101/2020.02.14.20023127
Lisotti, Mazzella, Su2037 effects of a fermented milk containing Kluyveromyces marxianus B0399 and Bifidobacterium Lactis BB12 in patients with irritable bowel syndrome: a new effective agent, Gastroenterology
Liu, Kuang, Li, Yang, Yan et al., Roles of the gut microbiota in severe SARS-CoV-2 infection, Cytokine Growth Factor Rev, doi:10.1016/j.cytogfr.2022.01.007
Maccaferri, Candela, Turroni, Centanni, Severgnini et al., IBS-associated phylogenetic unbalances of the intestinal microbiota are not reverted by probiotic supplementation, Gut Microbes, doi:10.4161/gmic.21009
Maccaferri, Klinder, Brigidi, Cavina, Costabile, Potential probiotic Kluyveromyces marxianus B0399 modulates the immune response in Caco-2 cells and peripheral blood mononuclear cells and impacts the human gut microbiota in an in vitro colonic model system, Appl Environ Microbiol, doi:10.1128/AEM.06385-11
Meng, Zhang, Cao, Yu, Fang et al., Gut dysbacteriosis and intestinal disease: mechanism and treatment, J Appl Microbiol, doi:10.1111/jam.14661
Page, Kell, Pretorius, The Role of Lipopolysaccharide-Induced Cell Signalling in Chronic Inflammation, Chronic Stress, doi:10.1177/24705470221076390
Quarella, Lovrovich, Scalabrin, Campedelli, Backovic et al., Draft Genome Sequence of the Probiotic Yeast Kluyveromyces marxianus fragilis B0399, Genome Announc, doi:10.1128/genomeA.00923-16
Rodriguez, Bifano, Roca Goma, Plasencia, Torralba et al., Effect and Tolerability of a Nutritional Supplement Based on a Synergistic Combination of beta-Glucans and Selenium-and Zinc-Enriched Saccharomyces cerevisiae (ABB C1((R))) in Volunteers Receiving the Influenza or the COVID-19 Vaccine: A Randomized, Double-Blind, Placebo-Controlled Study, Nutrients, doi:10.3390/nu13124347
Sanders, Merenstein, Reid, Gibson, Rastall, Probiotics and prebiotics in intestinal health and disease: from biology to the clinic, Nat Rev Gastroenterol Hepatol, doi:10.1038/s41575-019-0173-3
Singh, Sharma, Lee, Yadav, SARS-CoV-2: Recent Variants and Clinical Efficacy of Antibody-Based Therapy, Front Cell Infect Microbiol, doi:10.3389/fcimb.2022.839170
Theodorakopoulou, Alexandrou, Boutou, Ferro, Ortiz et al., Renin-angiotensin system blockers during the COVID-19 pandemic: an update for patients with hypertension and chronic kidney disease, Clin Kidney J, doi:10.1093/ckj/sfab272
Wang, Wang, Sun, Ren, Zhu et al., Potential Associations Between Microbiome and COVID-19, Front Med, doi:10.3389/fmed.2021.785496
Willers, Viemann, Role of the gut microbiota in airway immunity and host defense against respiratory infections, Biol Chem, doi:10.1515/hsz-2021-0281
Xu, Li, Zhu, Liang, Fang et al., Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding, Nat Med, doi:10.1038/s41591-020-0817-4
Zhang, Wang, Chen, Interactions between Intestinal Microflora/Probiotics and the Immune System, Biomed Res Int, doi:10.1155/2019/6764919
DOI record:
{
"DOI": "10.1016/j.medmic.2022.100061",
"ISSN": [
"2590-0978"
],
"URL": "http://dx.doi.org/10.1016/j.medmic.2022.100061",
"alternative-id": [
"S2590097822000118"
],
"article-number": "100061",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Oral intake of Kluyveromyces marxianus B0399 plus lactobacillus rhamnosus CECT 30579 to mitigate symptoms in COVID-19 patients: A randomized open label clinical trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Medicine in Microecology"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.medmic.2022.100061"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 The Author(s). Published by Elsevier B.V."
}
],
"author": [
{
"affiliation": [],
"family": "Navarro-López",
"given": "Vicente",
"sequence": "first"
},
{
"affiliation": [],
"family": "Hernández-Belmonte",
"given": "Adriana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pérez Soto",
"given": "Maria Isabel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ayo-González",
"given": "Maikel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Losa-Rodríguez",
"given": "Guillermo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ros-Sánchez",
"given": "Esther",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martínez-Gabarrón",
"given": "Maravillas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sánchez-Pellicer",
"given": "Pedro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aguera-Santos",
"given": "Juan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Núñez-Delegido",
"given": "Eva",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ruzafa-Costas",
"given": "Beatriz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Picó-Monllor",
"given": "José Antonio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Navarro-Moratalla",
"given": "Laura",
"sequence": "additional"
}
],
"container-title": "Medicine in Microecology",
"container-title-short": "Medicine in Microecology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
8,
24
]
],
"date-time": "2022-08-24T05:52:52Z",
"timestamp": 1661320372000
},
"deposited": {
"date-parts": [
[
2022,
8,
24
]
],
"date-time": "2022-08-24T05:52:53Z",
"timestamp": 1661320373000
},
"indexed": {
"date-parts": [
[
2022,
8,
24
]
],
"date-time": "2022-08-24T06:14:47Z",
"timestamp": 1661321687677
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
8
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
1
]
],
"date-time": "2022-08-01T00:00:00Z",
"timestamp": 1659312000000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 17,
"start": {
"date-parts": [
[
2022,
8,
18
]
],
"date-time": "2022-08-18T00:00:00Z",
"timestamp": 1660780800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2590097822000118?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2590097822000118?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "100061",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
8
]
]
},
"published-print": {
"date-parts": [
[
2022,
8
]
]
},
"publisher": "Elsevier BV",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2590097822000118"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Gastroenterology",
"Medicine (miscellaneous)",
"Rheumatology",
"Immunology and Allergy"
],
"subtitle": [],
"title": "Oral intake of Kluyveromyces marxianus B0399 plus lactobacillus rhamnosus CECT 30579 to mitigate symptoms in COVID-19 patients: A randomized open label clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}
