Clinical Evaluation of Umifenovir as a Potential Antiviral Therapy for COVID-19: A Multi-center, Randomized, Controlled Clinical Trial
et al., Oman Medical Journal, doi:10.5001/omj.2025.51, IRCT20080901001165N46, Dec 2024
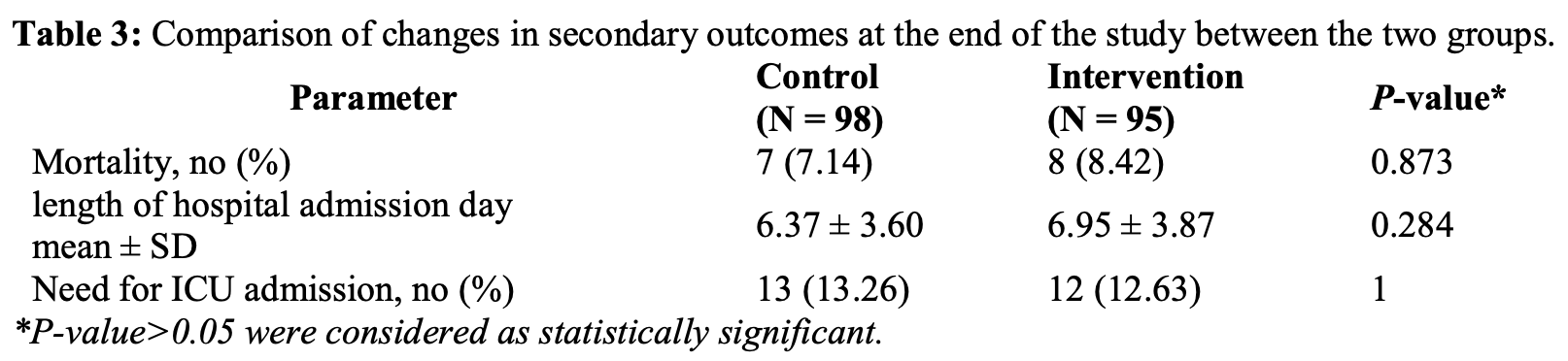
RCT 193 hospitalized COVID-19 patients showing no significant difference in mortality, ICU admission, or hospitalization with umifenovir treatment.
|
risk of death, 17.9% higher, RR 1.18, p = 0.79, treatment 8 of 95 (8.4%), control 7 of 98 (7.1%).
|
|
risk of ICU admission, 4.8% lower, RR 0.95, p = 1.00, treatment 12 of 95 (12.6%), control 13 of 98 (13.3%), NNT 158.
|
|
hospitalization time, 9.1% higher, relative time 1.09, p = 0.28, treatment mean 6.95 (±3.87) n=95, control mean 6.37 (±3.6) n=98.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kianpour et al., 31 Dec 2024, Randomized Controlled Trial, Iran, peer-reviewed, mean age 56.2, 13 authors, study period April 2020 - March 2021, trial IRCT20080901001165N46.
Contact: farhad.najm@gmail.com.
Clinical Evaluation of Umifenovir as a Potential Antiviral Therapy for COVID-19: A Multi-center, Randomized, Controlled Clinical Trial
Oman Medical Journal, doi:10.5001/omj.2025.51
Objectives: To evaluate the efficacy and safety of umifenovir as a potential antiviral therapy for COVID-19. This study aims to determine whether umifenovir can improve clinical outcomes, reduce hospitalization duration, and enhance recovery rates in patients diagnosed with COVID-19 compared to standard care.
Methods: A multicenter, open-label, randomized controlled trial was conducted involving 260 patients diagnosed with COVID-19. Participants were randomly assigned to receive either umifenovir (200 mg every six hours for seven days) or standard care. The primary outcome was clinical improvement assessed via the New Early Warning Signs 2 (NEWS-2) scoring system, while secondary outcomes included changes in CT scan scores, length of hospital stay, intensive care unit (ICU) admission rates, and mortality. Results: Of the 260 enrolled patients, 193 completed the study. Both groups showed significant reductions in clinical symptoms, but myalgia was more prevalent in the umifenovir group. The intervention group demonstrated a significant decrease in CT scan scores; however, there were no significant differences in hospital stay duration, ICU admissions, or mortality rates between groups. Conclusions: While umifenovir exhibited some immunological benefits in COVID-19 patients, it did not significantly improve broader patient-important outcomes compared to standard care. Therefore, its use in clinical practice for COVID-19 treatment is currently not justified, highlighting the need for further research to explore alternative therapeutic strategies.
Disclosure The authors declare that there is no conflict of interest regarding this publication. Part of the study, which was run at Sina Hospital, was funded by the Tehran University of Medical Sciences, grant number 99-1-104-47199.
References
Australia, How to treat mild COVID-19 symptoms and who can have oral antiviral treatments for COVID-19
Balkhair, COVID-19 Pandemic: A New Chapter in the History of Infectious Diseases, Oman Med J
Baloch, Baloch, Zheng, The Coronavirus Disease 2019 (COVID-19) Pandemic, Tohoku J Exp Med
Deng, Li, Zeng, Liu, Li et al., Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study, J Infect
Dhama, Sharun, Tiwari, Dadar, Malik et al., COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics, Hum Vaccin Immunother
Francone, Iafrate, Masci, Coco, Cilia et al., Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis, Eur Radiol
Glushkov, Ta, Liu, Nikolaeva, Mekhanizmy immunemoduliruiushchego deĭstviia arbidola [Mechanisms of arbidole's immunomodulating action, Vestn Ross Akad Med Nauk
Harrison, Lin, Wang, Mechanisms of SARS-CoV-2 Transmission and Pathogenesis, Trends Immunol
Huang, Yu, Wang, Yang, Yao et al., Efficacy and safety of umifenovir for coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis, J Med Virol
Jie, Hongmei, Ping, Kuikui, Bohan et al., Beneficial effect of Arbidol in the management of COVID-19 infection, Aging
Jones, NEWSDIG: The National Early Warning Score Development and Implementation Group, Clin Med (Lond)
Muralidar, Ambi, Sekaran, Krishnan, The emergence of COVID-19 as a global pandemic: Understanding the epidemiology, immune response and potential therapeutic targets of SARS-CoV-2, Biochimie
Ni, Yang, Yang, Bao, Li et al., Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19, Crit Care
Pshenichnaya, Bulgakova, Lvov, Poromov, Selkova et al., Clinical efficacy of umifenovir in influenza and ARVI (study ARBITR), Ter Arkh
Wang, Fan, Salam, Horby, Hayden et al., Comparative Effectiveness of Combined Favipiravir and Oseltamivir Therapy Versus Oseltamivir Monotherapy in Critically Ill Patients With Influenza Virus Infection, J Infect Dis
Wang, Yang, Li, Wen, Zhang, Clinical Features of 69 Cases With Coronavirus Disease 2019 in Wuhan, China, Clin Infect Dis
Wu, Wang, Kuo, Shannar, Peter et al., An Update on Current Therapeutic Drugs Treating COVID-19, Curr Pharmacol Rep
Xudan, Yuying, Baoyi, Jianwen, Wenxin et al., Association of clinical characteristics and antiviral drugs with viral RNA clearance in patients with COVID-19 in Guangzhou, China: a retrospective cohort study, medRxiv
DOI record:
{
"DOI": "10.5001/omj.2025.51",
"ISSN": [
"1999-768X",
"2070-5204"
],
"URL": "http://dx.doi.org/10.5001/omj.2025.51",
"author": [
{
"affiliation": [],
"family": "Kianpour",
"given": "Parisa",
"sequence": "first"
},
{
"affiliation": [],
"family": "Mourtami",
"given": "Reza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sahab-Negah",
"given": "Sajad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Panahi",
"given": "Yunes",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bayatani",
"given": "Behnam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Qazivini",
"given": "Ali",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Izadi",
"given": "Morteza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mojtaheszadeh",
"given": "Mojtaba",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shahrami",
"given": "Bita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hadadi",
"given": "Azar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Montazeri",
"given": "Mahnaz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bagher",
"given": "Negin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Najmeddin",
"given": "Farhad",
"sequence": "additional"
}
],
"container-title": "Oman Medical Journal",
"container-title-short": "Oman Med J",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
12,
10
]
],
"date-time": "2024-12-10T07:05:16Z",
"timestamp": 1733814316000
},
"deposited": {
"date-parts": [
[
2024,
12,
10
]
],
"date-time": "2024-12-10T07:05:16Z",
"timestamp": 1733814316000
},
"indexed": {
"date-parts": [
[
2024,
12,
11
]
],
"date-time": "2024-12-11T05:21:48Z",
"timestamp": 1733894508710,
"version": "3.30.1"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024
]
]
},
"member": "2907",
"original-title": [],
"prefix": "10.5001",
"published": {
"date-parts": [
[
2024
]
]
},
"published-online": {
"date-parts": [
[
2024
]
]
},
"publisher": "Oman Medical Journal",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://www.omjournal.org/articleDetails.aspx?coType=2&aId=3789"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Clinical Evaluation of Umifenovir as a Potential Antiviral Therapy for COVID-19: A Multi-center, Randomized, Controlled Clinical Trial",
"type": "journal-article"
}