Convalescent Plasma Treatment in Patients with Covid-19: A Systematic Review and Meta-Analysis
et al., Frontiers in Immunology, doi:10.3389/fimmu.2022.817829, Feb 2022
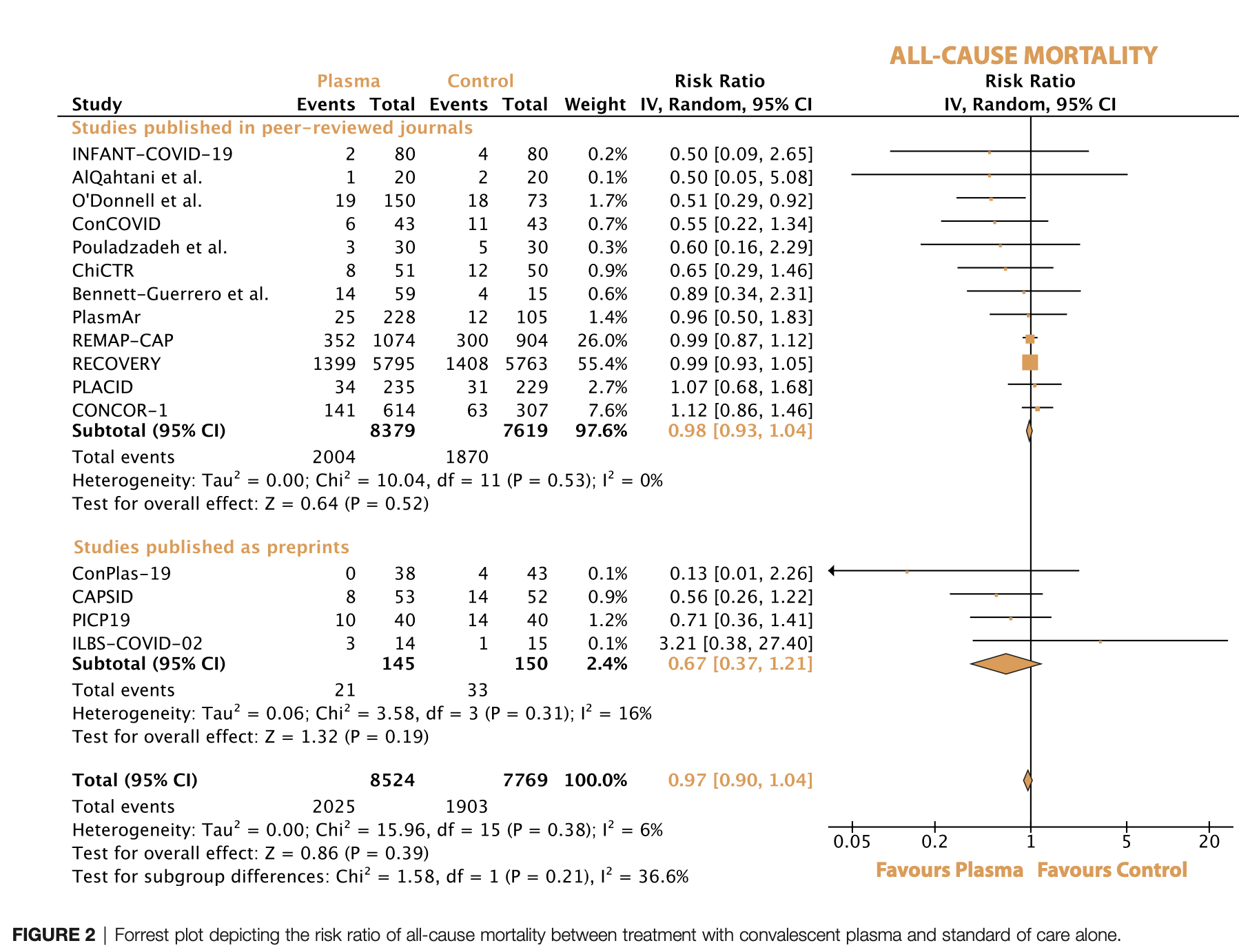
Meta-analysis of 16 RCTs with 16,317 COVID-19 patients showing no significant difference in mortality, mechanical ventilation, time to clinical improvement, or time to discharge with convalescent plasma treatment. Subgroup analyses in critically ill, non-critically ill, and seronegative patients also showed no significant difference in mortality. Authors note that the high certainty evidence does not support routine use of convalescent plasma in COVID-19 patients.
Currently there are 58 convalescent plasma studies and meta-analysis shows:
| Outcome | Improvement |
|---|---|
| Mortality | 3% higher [-2‑7%] |
| Ventilation | 0% higher [-11‑14%] |
| ICU admission | 9% higher [-5‑26%] |
| Hospitalization | 2% higher [-11‑16%] |
| Cases | 40% more [-42‑237%] |
Jorda et al., 7 Feb 2022, peer-reviewed, 7 authors.
Contact: georg.gelbenegger@meduniwien.ac.at.
Convalescent Plasma Treatment in Patients with Covid-19: A Systematic Review and Meta-Analysis
Frontiers in Immunology, doi:10.3389/fimmu.2022.817829
Convalescent plasma is a suggested treatment for Coronavirus disease 2019 , but its efficacy is uncertain. We aimed to evaluate whether the use of convalescent plasma is associated with improved clinical outcomes in patients with Covid-19.In this systematic review and meta-analysis, we searched randomized controlled trials investigating the use of convalescent plasma in patients with Covid-19 in Medline, Embase, Web of Science, Cochrane Library, and medRxiv from inception to October 17 th , 2021. Two reviewers independently extracted the data. The primary efficacy outcome was all-cause mortality. The Cochrane Risk of Bias Tool and GRADE (Grading of Recommendations Assessment, Development and Evaluation) method were used. This study was registered with PROSPERO, CRD42021284861. Of the 8874 studies identified in the initial search, sixteen trials comprising 16 317 patients with Covid-19 were included. In the overall population, the all-cause mortality was 23.8% (2025 of 8524) with convalescent plasma and 24.4% (1903 of 7769) with standard of care (risk ratio (RR) 0.97, 95% CI 0.90-1.04) (high-certainty evidence). All-cause mortality did not differ in the subgroups of noncritically ill (21.7% [1288 of 5929] vs. 22.4% [1320 of 5882]) and critically ill (36.9% [518 of 1404] vs. 36.4% [455 of 1247]) patients with Covid-19. The use of convalescent plasma in patients who tested negative for anti-SARS-CoV-2 antibodies at baseline was not associated with significantly improved survival (RR 0.94, 95% CI 0.87-1.02). In the overall study population, initiation of mechanical ventilation (RR 0.97, 95% CI 0.88-1.07), time to clinical improvement (HR 1.09, 95% CI 0.91-1.30), and time to discharge (HR 0.95, 95% CI 0.89-1.02) were similar between the two groups. In patients with Covid-19, treatment with convalescent plasma, as compared with control, was not associated with lower all-cause mortality or improved disease progression, irrespective of disease severity and baseline antibody status. Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier PROSPERO (CRD42021284861).
AUTHOR CONTRIBUTIONS GG conceived the idea. AJ and GG performed the research, interpreted the results, and drafted the manuscript. All authors critically revised the manuscript and approved the final version for publication.
SUPPLEMENTARY MATERIAL The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.817829/ full#supplementary-material Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Publisher's Note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agarwal, Mukherjee, Kumar, Chatterjee, Bhatnagar et al., Convalescent Plasma in the Management of Moderate Covid-19 in Adults in India: Open Label Phase II Multicentre Randomised Controlled Trial (PLACID Trial), Bmj, doi:10.1136/bmj.m3939
Alqahtani, Abdulrahman, Almadani, Alali, Zamrooni et al., Randomized Controlled Trial of Convalescent Plasma Therapy Against Standard Therapy in Patients With Severe COVID-19
Avendaño-Solà, Ramos-Martıńez, Muñez-Rubio, Ruiz-Antorań, De Molina et al., Convalescent Plasma for COVID-19: A Multicenter, Randomized Clinical Trial, medRxiv, doi:10.1101/2020.08.26.20182444
Bajpai, Kumar, Maheshwari, Chhabra, Gupta, Efficacy of Convalescent Plasma Therapy Compared to Fresh Frozen Plasma in Severely Ill COVID-19 Patients: A Pilot Randomized Controlled Trial, medRxiv, doi:10.1101/2020.10.25.20219337
Bennett-Guerrero, Romeiser, Talbot, Ahmed, Mamone et al., Severe Acute Respiratory Syndrome Coronavirus 2 Convalescent Plasma Versus Standard Plasma in Coronavirus Disease 2019 Infected Hospitalized Patients in New York: A Double-Blind Randomized Trial, Crit Care Med, doi:10.1097/CCM.0000000000005066
Beǵin, Callum, Jamula, Cook, Heddle et al., Convalescent Plasma for Hospitalized Patients With COVID-19: An Open-Label, Randomized Controlled Trial, Nat Med, doi:10.1038/s41591-021-01488-2
Chen, Xiong, Bao, Shi, Convalescent Plasma as a Potential Therapy for COVID-19, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30141-9
Clark, Guilpain, Filip, Pansu, Bihan et al., Convalescent Plasma for Persisting COVID-19 Following Therapeutic Lymphocyte Depletion: A Report of Rapid Recovery, Br J Haematol, doi:10.1111/bjh.16981
Dougan, Nirula, Azizad, Mocherla, Gottlieb et al., Bamlanivimab Plus Etesevimab in Mild or Moderate Covid-19, N Engl J Med, doi:10.1056/NEJMoa2102685
Estcourt, Turgeon, Mcquilten, Mcverry, Al-Beidh et al., Effect of Convalescent Plasma on Organ Support-Free Days in Critically Ill Patients With COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2021.18178
Gharbharan, Jordans, Geurtsvankessel, Hollander, Karim et al., Effects of Potent Neutralizing Antibodies From Convalescent Plasma in Patients Hospitalized for Severe SARS-CoV-2
Gupta, Gonzalez-Rojas, Juarez, Casal, Moya et al., Early Treatment for Covid-19 With SARS-CoV-2 Neutralizing Antibody Sotrovimab, N Engl J Med, doi:10.1101/2021.05.27.21257096
Guyatt, Oxman, Vist, Kunz, Falck-Ytter et al., Clinical Efficacy of Convalescent Plasma for Treatment of COVID-19 Infections: Results of a Multicenter Clinical Study, Transfus Apher Sci, doi:10.1016/j.transci.2020.102875
Horby, Mafham, Peto, Campbell, Pessoa-Amorim, Casirivimab and Imdevimab in Patients Admitted to Hospital With COVID-19 (RECOVERY): A Randomised, Controlled, Open-Label, Platform Trial. medRxiv, doi:10.1101/2021.06.15.21258542
Hueso, Pouderoux, Pere, Beaumont, Raillon et al., Convalescent Plasma Therapy for B-Cell-Depleted Patients With Protracted COVID-19, Blood, doi:10.1182/blood.2020008423
Janiaud, Axfors, Schmitt, Gloy, Ebrahimi et al., Association of Convalescent Plasma Treatment With Clinical Outcomes in Patients With COVID-19: A Systematic Review and Meta-Analysis, JAMA, doi:10.1001/jama.2021.2747
Joyner, Bruno, Klassen, Kunze, Johnson et al., Safety Update: COVID-19 Convalescent Plasma in 20,000 Hospitalized Patients, Mayo Clin Proc, doi:10.1016/j.mayocp.2020.06.028
Joyner, Carter, Senefeld, Klassen, Mills et al., Convalescent Plasma Antibody Levels and the Risk of Death From Covid-19, N Engl J Med, doi:10.1056/NEJMoa2031893
Joyner, Senefeld, Klassen, Mills, Johnson et al., Effect of Convalescent Plasma on Mortality Among Hospitalized Patients With COVID-19: Initial Three-Month Experience, medRxiv, doi:10.1101/2020.08.12.20169359
Körper, Weiss, Zickler, Wiesmann, Zacharowski et al., High Dose Convalescent Plasma in COVID-19: Results From the Randomized Trial CAPSID, medRxiv, doi:10.1101/2021.05.10.21256192
Ledford, COVID Antibody Treatments Show Promise for Preventing Severe Disease, Nature, doi:10.1038/d41586-021-00650-7
Li, Zhang, Hu, Tong, Zheng et al., Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-Threatening COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2020.10044
Libster, Marc, Wappner, Coviello, Bianchi et al., Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults, N Engl J Med, doi:10.1056/NEJMoa2033700
Luke, Kilbane, Jackson, Hoffman, Meta-Analysis: Convalescent Blood Products for Spanish Influenza Pneumonia: A Future H5N1 Treatment?, Ann Intern Med, doi:10.7326/0003-4819-145-8-200610170-00139
Mcgowan, Sampson, Salzwedel, Cogo, Foerster et al., PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement, J Clin Epidemiol, doi:10.1016/j.jclinepi.2016.01.021
O'donnell, Grinsztejn, Cummings, Justman, Lamb et al., A Randomized Double-Blind Controlled Trial of Convalescent Plasma in Adults With Severe COVID-19, J Clin Invest, doi:10.1172/JCI150646
Page, Mckenzie, Bossuyt, Boutron, Hoffmann et al., The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews, BMJ, doi:10.1136/bmj.n71
Pouladzadeh, Safdarian, Eshghi, Abolghasemi, Bavani et al., A Randomized Clinical Trial Evaluating the Immunomodulatory Effect of Convalescent Plasma on COVID-19-Related Cytokine Storm, Intern Emerg Med, doi:10.1007/s11739-021-02734-8
Ray, Paul, Bandopadhyay, 'rozario, Sarif et al., Clinical and Immunological Benefits of Convalescent Plasma Therapy in Severe COVID-19: Insights From a Single Center Open Label Randomised Control Trial, medRxiv, doi:10.1101/2020.11.25.20237883
Rodionov, Biener, Spieth, Achleitner, Holig et al., Potential Benefit of Convalescent Plasma Transfusions in Immunocompromised Patients With COVID-19, Lancet Microbe, doi:10.1016/S2666-5247(21)00030-6
Shankar-Hari, Vale, Godolphin, Fisher, Higgins et al., Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-Analysis, JAMA, doi:10.1001/jama.2021.11330
Simonovich, Pratx, Scibona, Beruto, Vallone et al., A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia, N Engl J Med, doi:10.1056/NEJMoa2031304
Sterne, Murthy, Diaz, Slutsky, Villar et al., Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-Analysis, JAMA, doi:10.1001/jama.2020.17023
Thompson, Henderson, Shah, Rubinstein, Joyner et al., Association of Convalescent Plasma Therapy With Survival in Patients With Hematologic Cancers and COVID-19, JAMA Oncol, doi:10.1101/2021.02.05.21250953
Zeitlinger, Idzko, Inhaled Budesonide for Early Treatment of COVID-19, Lancet Respir Med, doi:10.1016/S2213-2600(21)00215-0
DOI record:
{
"DOI": "10.3389/fimmu.2022.817829",
"ISSN": [
"1664-3224"
],
"URL": "http://dx.doi.org/10.3389/fimmu.2022.817829",
"abstract": "<jats:p>Convalescent plasma is a suggested treatment for Coronavirus disease 2019 (Covid-19), but its efficacy is uncertain. We aimed to evaluate whether the use of convalescent plasma is associated with improved clinical outcomes in patients with Covid-19.In this systematic review and meta-analysis, we searched randomized controlled trials investigating the use of convalescent plasma in patients with Covid-19 in Medline, Embase, Web of Science, Cochrane Library, and medRxiv from inception to October 17<jats:sup>th</jats:sup>, 2021. Two reviewers independently extracted the data. The primary efficacy outcome was all-cause mortality. The Cochrane Risk of Bias Tool and GRADE (Grading of Recommendations Assessment, Development and Evaluation) method were used. This study was registered with PROSPERO, CRD42021284861. Of the 8874 studies identified in the initial search, sixteen trials comprising 16 317 patients with Covid-19 were included. In the overall population, the all-cause mortality was 23.8% (2025 of 8524) with convalescent plasma and 24.4% (1903 of 7769) with standard of care (risk ratio (RR) 0.97, 95% CI 0.90-1.04) (high-certainty evidence). All-cause mortality did not differ in the subgroups of noncritically ill (21.7% [1288 of 5929] vs. 22.4% [1320 of 5882]) and critically ill (36.9% [518 of 1404] vs. 36.4% [455 of 1247]) patients with Covid-19. The use of convalescent plasma in patients who tested negative for anti-SARS-CoV-2 antibodies at baseline was not associated with significantly improved survival (RR 0.94, 95% CI 0.87-1.02). In the overall study population, initiation of mechanical ventilation (RR 0.97, 95% CI 0.88-1.07), time to clinical improvement (HR 1.09, 95% CI 0.91-1.30), and time to discharge (HR 0.95, 95% CI 0.89-1.02) were similar between the two groups. In patients with Covid-19, treatment with convalescent plasma, as compared with control, was not associated with lower all-cause mortality or improved disease progression, irrespective of disease severity and baseline antibody status.</jats:p><jats:sec><jats:title>Systematic Review Registration</jats:title><jats:p><jats:uri>https://www.crd.york.ac.uk/prospero/</jats:uri>, identifier PROSPERO (<jats:uri>CRD42021284861</jats:uri>).</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fimmu.2022.817829"
],
"author": [
{
"affiliation": [],
"family": "Jorda",
"given": "Anselm",
"sequence": "first"
},
{
"affiliation": [],
"family": "Kussmann",
"given": "Manuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kolenchery",
"given": "Nebu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Siller-Matula",
"given": "Jolanta M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zeitlinger",
"given": "Markus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jilma",
"given": "Bernd",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gelbenegger",
"given": "Georg",
"sequence": "additional"
}
],
"container-title": "Frontiers in Immunology",
"container-title-short": "Front. Immunol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2022,
2,
7
]
],
"date-time": "2022-02-07T06:57:35Z",
"timestamp": 1644217055000
},
"deposited": {
"date-parts": [
[
2022,
2,
7
]
],
"date-time": "2022-02-07T06:57:38Z",
"timestamp": 1644217058000
},
"indexed": {
"date-parts": [
[
2024,
9,
19
]
],
"date-time": "2024-09-19T16:18:20Z",
"timestamp": 1726762700601
},
"is-referenced-by-count": 30,
"issued": {
"date-parts": [
[
2022,
2,
7
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
7
]
],
"date-time": "2022-02-07T00:00:00Z",
"timestamp": 1644192000000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fimmu.2022.817829/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2022,
2,
7
]
]
},
"published-online": {
"date-parts": [
[
2022,
2,
7
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1016/S1473-3099(20)30141-9",
"article-title": "Convalescent Plasma as a Potential Therapy for COVID-19",
"author": "Chen",
"doi-asserted-by": "publisher",
"first-page": "398",
"journal-title": "Lancet Infect Dis",
"key": "B1",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.7326/0003-4819-145-8-200610170-00139",
"article-title": "Meta-Analysis: Convalescent Blood Products for Spanish Influenza Pneumonia: A Future H5N1 Treatment",
"author": "Luke",
"doi-asserted-by": "publisher",
"first-page": "599",
"journal-title": "Ann Intern Med",
"key": "B2",
"volume": "145",
"year": "2006"
},
{
"DOI": "10.1111/bjh.16981",
"article-title": "Convalescent Plasma for Persisting COVID-19 Following Therapeutic Lymphocyte Depletion: A Report of Rapid Recovery",
"author": "Clark",
"doi-asserted-by": "publisher",
"journal-title": "Br J Haematol",
"key": "B3",
"volume": "190",
"year": "2020"
},
{
"DOI": "10.1101/2021.02.05.21250953",
"article-title": "Association of Convalescent Plasma Therapy With Survival in Patients With Hematologic Cancers and COVID-19",
"author": "Thompson",
"doi-asserted-by": "publisher",
"journal-title": "JAMA Oncol",
"key": "B4",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1016/j.mayocp.2020.06.028",
"article-title": "Safety Update: COVID-19 Convalescent Plasma in 20,000 Hospitalized Patients",
"author": "Joyner",
"doi-asserted-by": "publisher",
"journal-title": "Mayo Clin Proc",
"key": "B5",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.1101/2020.08.12.20169359",
"article-title": "Effect of Convalescent Plasma on Mortality Among Hospitalized Patients With COVID-19: Initial Three-Month Experience",
"author": "Joyner",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "B6",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2031893",
"article-title": "Convalescent Plasma Antibody Levels and the Risk of Death From Covid-19",
"author": "Joyner",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B7",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1136/bmj.n71",
"article-title": "The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews",
"author": "Page",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "B8",
"volume": "372",
"year": "2021"
},
{
"DOI": "10.1016/j.jclinepi.2016.01.021",
"article-title": "PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement",
"author": "McGowan",
"doi-asserted-by": "publisher",
"journal-title": "J Clin Epidemiol",
"key": "B9",
"volume": "75",
"year": "2016"
},
{
"DOI": "10.1001/jama.2021.2747",
"article-title": "Association of Convalescent Plasma Treatment With Clinical Outcomes in Patients With COVID-19: A Systematic Review and Meta-Analysis",
"author": "Janiaud",
"doi-asserted-by": "publisher",
"journal-title": "JAMA",
"key": "B10",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1136/bmj.39489.470347.AD",
"article-title": "GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations",
"author": "Guyatt",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "B11",
"volume": "336",
"year": "2008"
},
{
"DOI": "10.1016/j.transci.2020.102875",
"article-title": "Clinical Efficacy of Convalescent Plasma for Treatment of COVID-19 Infections: Results of a Multicenter Clinical Study",
"author": "Abolghasemi",
"doi-asserted-by": "publisher",
"first-page": "102875",
"journal-title": "Transfus Apher Sci",
"key": "B12",
"volume": "59",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m3939",
"article-title": "Convalescent Plasma in the Management of Moderate Covid-19 in Adults in India: Open Label Phase II Multicentre Randomised Controlled Trial (PLACID Trial)",
"author": "Agarwal",
"doi-asserted-by": "publisher",
"journal-title": "Bmj",
"key": "B13",
"volume": "371",
"year": "2020"
},
{
"DOI": "10.1038/s41598-021-89444-5",
"article-title": "Randomized Controlled Trial of Convalescent Plasma Therapy Against Standard Therapy in Patients With Severe COVID-19 Disease",
"author": "AlQahtani",
"doi-asserted-by": "publisher",
"first-page": "9927",
"journal-title": "Sci Rep",
"key": "B14",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1038/s41591-021-01488-2",
"article-title": "Convalescent Plasma for Hospitalized Patients With COVID-19: An Open-Label, Randomized Controlled Trial",
"author": "Bégin",
"doi-asserted-by": "publisher",
"journal-title": "Nat Med",
"key": "B15",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.18178",
"article-title": "Effect of Convalescent Plasma on Organ Support-Free Days in Critically Ill Patients With COVID-19: A Randomized Clinical Trial",
"author": "Estcourt",
"doi-asserted-by": "publisher",
"journal-title": "JAMA",
"key": "B16",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1038/s41467-021-23469-2",
"article-title": "Effects of Potent Neutralizing Antibodies From Convalescent Plasma in Patients Hospitalized for Severe SARS-CoV-2 Infection",
"author": "Gharbharan",
"doi-asserted-by": "publisher",
"first-page": "3189",
"journal-title": "Nat Commun",
"key": "B17",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00897-7",
"article-title": "Convalescent Plasma in Patients Admitted to Hospital With COVID-19 (RECOVERY): A Randomised Controlled, Open-Label, Platform Trial",
"doi-asserted-by": "publisher",
"journal-title": "Lancet",
"key": "B18",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.10044",
"article-title": "Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-Threatening COVID-19: A Randomized Clinical Trial",
"author": "Li",
"doi-asserted-by": "publisher",
"journal-title": "JAMA",
"key": "B19",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2033700",
"article-title": "Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults",
"author": "Libster",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B20",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1172/JCI150646",
"article-title": "A Randomized Double-Blind Controlled Trial of Convalescent Plasma in Adults With Severe COVID-19",
"author": "O’Donnell",
"doi-asserted-by": "publisher",
"journal-title": "J Clin Invest",
"key": "B21",
"volume": "131",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2031304",
"article-title": "A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia",
"author": "Simonovich",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B22",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1097/CCM.0000000000005066",
"article-title": "Severe Acute Respiratory Syndrome Coronavirus 2 Convalescent Plasma Versus Standard Plasma in Coronavirus Disease 2019 Infected Hospitalized Patients in New York: A Double-Blind Randomized Trial",
"author": "Bennett-Guerrero",
"doi-asserted-by": "publisher",
"journal-title": "Crit Care Med",
"key": "B23",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1007/s11739-021-02734-8",
"article-title": "A Randomized Clinical Trial Evaluating the Immunomodulatory Effect of Convalescent Plasma on COVID-19-Related Cytokine Storm",
"author": "Pouladzadeh",
"doi-asserted-by": "publisher",
"journal-title": "Intern Emerg Med",
"key": "B24",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1101/2020.08.26.20182444",
"article-title": "Convalescent Plasma for COVID-19: A Multicenter, Randomized Clinical Trial",
"author": "Avendaño-Solà",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "B25",
"year": "2020"
},
{
"DOI": "10.1101/2020.10.25.20219337",
"article-title": "Efficacy of Convalescent Plasma Therapy Compared to Fresh Frozen Plasma in Severely Ill COVID-19 Patients: A Pilot Randomized Controlled Trial",
"author": "Bajpai",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "B26",
"year": "2020"
},
{
"DOI": "10.1101/2021.05.10.21256192",
"article-title": "High Dose Convalescent Plasma in COVID-19: Results From the Randomized Trial CAPSID",
"author": "Körper",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "B27",
"year": "2021"
},
{
"DOI": "10.1101/2020.11.25.20237883",
"article-title": "Clinical and Immunological Benefits of Convalescent Plasma Therapy in Severe COVID-19: Insights From a Single Center Open Label Randomised Control Trial",
"author": "Ray",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "B28",
"year": "2020"
},
{
"DOI": "10.1001/jama.2021.11330",
"article-title": "Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-Analysis",
"author": "Shankar-Hari",
"doi-asserted-by": "publisher",
"first-page": "499",
"journal-title": "JAMA",
"key": "B29",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.17023",
"article-title": "Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-Analysis",
"author": "Sterne",
"doi-asserted-by": "publisher",
"journal-title": "JAMA",
"key": "B30",
"volume": "324",
"year": "2020"
},
{
"key": "B31",
"volume-title": "Investigational COVID-19 Convalescent Plasma: Guidance for Industry",
"year": "2021"
},
{
"DOI": "10.1182/blood.2020008423",
"article-title": "Convalescent Plasma Therapy for B-Cell-Depleted Patients With Protracted COVID-19",
"author": "Hueso",
"doi-asserted-by": "publisher",
"journal-title": "Blood",
"key": "B32",
"volume": "136",
"year": "2020"
},
{
"DOI": "10.1016/S2666-5247(21)00030-6",
"article-title": "Potential Benefit of Convalescent Plasma Transfusions in Immunocompromised Patients With COVID-19",
"author": "Rodionov",
"doi-asserted-by": "publisher",
"first-page": "e138",
"journal-title": "Lancet Microbe",
"key": "B33",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00215-0",
"article-title": "Inhaled Budesonide for Early Treatment of COVID-19",
"author": "Zeitlinger",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Respir Med",
"key": "B34",
"volume": "9",
"year": "2021"
},
{
"key": "B35",
"unstructured": "COVID-19 Treatment Guidelines Panel 20212021"
},
{
"key": "B36",
"unstructured": "2021"
},
{
"DOI": "10.1038/d41586-021-00650-7",
"article-title": "COVID Antibody Treatments Show Promise for Preventing Severe Disease",
"author": "Ledford",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B37",
"volume": "591",
"year": "2021"
},
{
"DOI": "10.1101/2021.06.15.21258542",
"article-title": "Casirivimab and Imdevimab in Patients Admitted to Hospital With COVID-19 (RECOVERY): A Randomised, Controlled, Open-Label, Platform Trial",
"author": "Horby",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "B38",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2102685",
"article-title": "Bamlanivimab Plus Etesevimab in Mild or Moderate Covid-19",
"author": "Dougan",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B39",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1101/2021.05.27.21257096",
"article-title": "Early Treatment for Covid-19 With SARS-CoV-2 Neutralizing Antibody Sotrovimab",
"author": "Gupta",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B40",
"volume": "385",
"year": "2021"
}
],
"reference-count": 40,
"references-count": 40,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fimmu.2022.817829/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Convalescent Plasma Treatment in Patients with Covid-19: A Systematic Review and Meta-Analysis",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "13"
}