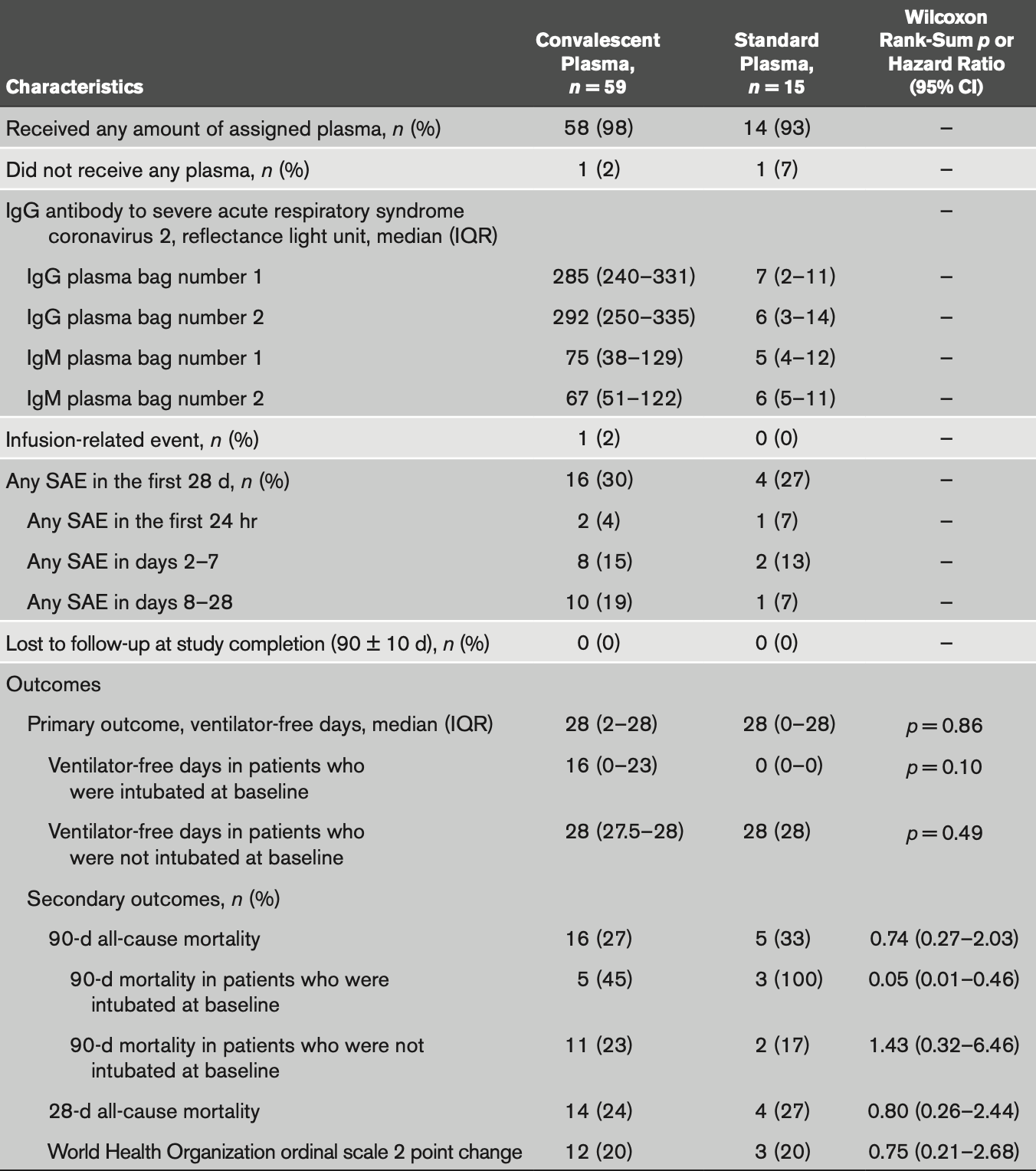
Severe Acute Respiratory Syndrome Coronavirus 2 Convalescent Plasma Versus Standard Plasma in Coronavirus Disease 2019 Infected Hospitalized Patients in New York
et al., Critical Care Medicine, doi:10.1097/CCM.0000000000005066, NCT04344535, Apr 2021
RCT 74 hospitalized patients in the USA, showing no significant difference with convalescent plasma treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
|
risk of death, 18.6% lower, RR 0.81, p = 0.75, treatment 16 of 59 (27.1%), control 5 of 15 (33.3%), NNT 16, day 90.
|
|
risk of death, 11.0% lower, RR 0.89, p = 1.00, treatment 14 of 59 (23.7%), control 4 of 15 (26.7%), NNT 34, day 28.
|
|
risk of no improvement, 0.4% lower, RR 1.00, p = 1.00, treatment 47 of 59 (79.7%), control 12 of 15 (80.0%), NNT 295.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Bennett-Guerrero et al., 16 Apr 2021, Double Blind Randomized Controlled Trial, USA, peer-reviewed, 18 authors, study period 8 April, 2020 - 1 February, 2021, average treatment delay 9.0 days, trial NCT04344535 (history).
Severe Acute Respiratory Syndrome Coronavirus 2 Convalescent Plasma Versus Standard Plasma in Coronavirus Disease 2019 Infected Hospitalized Patients in New York
Critical Care Medicine, doi:10.1097/ccm.0000000000005066
OBJECTIVES: Four peer-reviewed publications have reported results from randomized controlled trials of convalescent plasma for coronavirus disease 2019 infection; none were conducted in the United States nor used standard plasma as a comparator. To determine if administration of convalescent plasma to patients with coronavirus disease 2019 increases antibodies to severe acute respiratory syndrome coronavirus 2 and improves outcome. DESIGN: Double-blind randomized controlled trial. SETTING: Hospital in New York. PATIENTS: Patients with polymerase chain reaction documented coronavirus disease 2019 infection. INTERVENTIONS: Patients were randomized (4:1) to receive 2 U of convalescent plasma versus standard plasma. Antibodies to severe acute respiratory syndrome coronavirus 2 were measured in plasma units and in trial recipients.
MEASUREMENTS AND MAIN RESULTS: Enrollment was terminated after emergency use authorization was granted for convalescent plasma. Seventy-four patients were randomized. At baseline, mean (sd) Acute Physiology and Chronic Health Evaluation II score (23.4 [5.6] and 22.5 [6.6]), percent of patients intubated (19% and 20%), and median (interquartile range) days from symptom onset to randomization of 9 (6-18) and 9 (6-15), were similar in the convalescent plasma versus standard plasma arms, respectively. Convalescent plasma had high neutralizing activity (median [interquartile range] titer 1:526 [1:359-1:786]) and its administration increased antibodies to severe acute respiratory syndrome coronavirus 2 by 14.4%, whereas standard plasma administration led to an 8.6% decrease (p = 0.005). No difference was observed for ventilator-free days through 28 days (primary study endpoint): median (interquartile range) of 28 (2-28) versus 28 (0-28; p = 0.86) for the convalescent plasma and standard plasma groups, respectively. A greater than or equal to 2 point improvement in the World Health Organization scale was achieved by 20% of subjects in both arms (p = 0.99). All-cause mortality through 90 days was numerically lower in the convalescent plasma versus standard plasma groups (27% vs 33%; p = 0.63) but did not achieve statistical significance. A key prespecified subgroup analysis of time to death in patients who were intubated at baseline was statistically significant; however, sample size numbers were small. CONCLUSIONS: Administration of convalescent plasma to hospitalized patients with coronavirus disease 2019 infection increased antibodies to severe acute respiratory syndrome coronavirus disease 2 but was not associated with improved outcome.
References
Agarwal, Mukherjee, Kumar, PLACID Trial Collaborators: Convalescent plasma in the management of moderate Covid-19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID Trial), BMJ
Beigel, Aga, Mc, IRC005 Study Team: Anti-influenza immune plasma for the treatment of patients with severe influenza A: A randomised, double-blind, phase 3 trial, Lancet Respir Med
Brill, Daley, Gearwar, Generale, Halper et al., Stony Brook Medicine COVID Plasma Trial Group are as follows: Investigators: Elliott Bennett-Guerrero (Principal Investigator, Critical Care)
Carter, Freedenberg, Romeiser, Stony Brook Medicine COVID Plasma Trial Group: Impact of serological and PCR testing requirements on the selection of COVID-19 convalescent plasma donors, Transfusion
Dong, Du, Gardner, An interactive web-based dashboard to track COVID-19 in real time, Lancet Infect Dis
Duan, Liu, Li, Effectiveness of convalescent plasma therapy in severe COVID-19 patients, Proc Natl Acad Sci U S A
Fernando, Seshan, Pham ; Lillian, Browne, Carter et al., Plaque Reduction Neutralization Assay: Janet Hearing. Regulatory (Investigational New Drug [IND] and Institutional Review Board [IRB] support)
Freedenberg, Pan, Diehl, Neutralizing activity to SARS-CoV-2 of convalescent and control plasma used in a randomized controlled trial, Transfusion
Jin, Gu, Yuan, Treatment of six COVID-19 patients with convalescent plasma, medRxiv
Joyner, Bruno, Klassen, Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients, Mayo Clin Proc
Joyner, Expanded Access to Convalescent Plasma for the Treatment of Patients with COVID-19 protocol
Joyner, Senefeld, Klassen, US EAP COVID-19 Plasma Consortium: Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: Initial threemonth experience, medRxiv
Joyner, Wright, Fairweather, Early safety indicators of COVID-19 convalescent plasma in 5000 patients, J Clin Invest
Li, Zhang, Hu, Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: A randomized clinical trial, JAMA
Libster, Marc, Wappner, Fundación INFANT-COVID-19 Group: Early high-titer plasma therapy to prevent severe COVID-19 in older adults, N Engl J Med
Liu, Lin, Baine, Convalescent plasma treatment of severe COVID-19: A propensity score-matched control study, Nat Med
Mair-Jenkins, Saavedra-Campos, Baillie, Convalescent Plasma Study Group: The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis, J Infect Dis
Marano, Vaglio, Pupella, Convalescent plasma: New evidence for an old therapeutic tool?, Blood Transfus
Rogers, Shehadeh, Mylona, Convalescent plasma for patients with severe COVID-19: A matched cohort study, Clin Infect Dis
Salazar, Christensen, Graviss, Treatment of COVID-19 patients with convalescent plasma reveals a signal of significantly decreased mortality, Am J Pathol
Salazar, Perez, Ashraf, Treatment of coronavirus disease 2019 (COVID-19) patients with convalescent plasma, Am J Pathol
Sanders, Monogue, Jodlowski, Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review, JAMA
Shen, Wang, Zhao, Treatment of 5 critically ill patients with COVID-19 with convalescent plasma, JAMA
Simonovich, Pratx, Scibona, PlasmAr Study Group: A randomized trial of convalescent plasma in COVID-19 severe pneumonia, N Engl J Med
Ye, Fu, Ren, Treatment with convalescent plasma for COVID-19 patients in Wuhan, China, J Med Virol
DOI record:
{
"DOI": "10.1097/ccm.0000000000005066",
"ISSN": [
"0090-3493"
],
"URL": "http://dx.doi.org/10.1097/CCM.0000000000005066",
"author": [
{
"affiliation": [],
"family": "Bennett-Guerrero",
"given": "Elliott",
"sequence": "first"
},
{
"affiliation": [],
"family": "Romeiser",
"given": "Jamie L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Talbot",
"given": "Lillian R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahmed",
"given": "Tahmeena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mamone",
"given": "Linda J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singh",
"given": "Sunitha M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hearing",
"given": "Janet C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salman",
"given": "Huda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Holiprosad",
"given": "Dishaw D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Freedenberg",
"given": "Alex T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carter",
"given": "Jason A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Browne",
"given": "Nicholas J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cosgrove",
"given": "Megan E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shevik",
"given": "Margaret E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Generale",
"given": "Laura M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Andrew",
"given": "Margaret A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nachman",
"given": "Sharon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fries",
"given": "Bettina C.",
"sequence": "additional"
}
],
"container-title": "Critical Care Medicine",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
4,
20
]
],
"date-time": "2021-04-20T03:19:51Z",
"timestamp": 1618888791000
},
"deposited": {
"date-parts": [
[
2021,
6,
21
]
],
"date-time": "2021-06-21T06:00:29Z",
"timestamp": 1624255229000
},
"indexed": {
"date-parts": [
[
2022,
12,
21
]
],
"date-time": "2022-12-21T02:36:13Z",
"timestamp": 1671590173033
},
"is-referenced-by-count": 31,
"issued": {
"date-parts": [
[
2021,
4,
16
]
]
},
"language": "en",
"link": [
{
"URL": "https://journals.lww.com/10.1097/CCM.0000000000005066",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "276",
"original-title": [],
"prefix": "10.1097",
"published": {
"date-parts": [
[
2021,
4,
16
]
]
},
"published-print": {
"date-parts": [
[
2021,
4,
16
]
]
},
"publisher": "Ovid Technologies (Wolters Kluwer Health)",
"reference": [
{
"DOI": "10.1016/S1473-3099(20)30120-1",
"article-title": "An interactive web-based dashboard to track COVID-19 in real time.",
"author": "Dong",
"doi-asserted-by": "crossref",
"first-page": "533",
"journal-title": "Lancet Infect Dis",
"key": "R1-20210618",
"volume": "20",
"year": "2020"
},
{
"article-title": "Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review.",
"author": "Sanders",
"first-page": "1824",
"journal-title": "JAMA",
"key": "R2-20210618",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1093/infdis/jiu396",
"article-title": "The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis.",
"author": "Mair-Jenkins",
"doi-asserted-by": "crossref",
"first-page": "80",
"journal-title": "J Infect Dis",
"key": "R3-20210618",
"volume": "211",
"year": "2015"
},
{
"article-title": "Convalescent plasma: New evidence for an old therapeutic tool?",
"author": "Marano",
"first-page": "152",
"journal-title": "Blood Transfus",
"key": "R4-20210618",
"volume": "14",
"year": "2016"
},
{
"DOI": "10.1073/pnas.2004168117",
"article-title": "Effectiveness of convalescent plasma therapy in severe COVID-19 patients.",
"author": "Duan",
"doi-asserted-by": "crossref",
"first-page": "9490",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "R5-20210618",
"volume": "117",
"year": "2020"
},
{
"article-title": "Treatment of six COVID-19 patients with convalescent plasma.",
"author": "Jin",
"journal-title": "medRxiv",
"key": "R6-20210618"
},
{
"DOI": "10.1016/j.ajpath.2020.05.014",
"article-title": "Treatment of coronavirus disease 2019 (COVID-19) patients with convalescent plasma.",
"author": "Salazar",
"doi-asserted-by": "crossref",
"first-page": "1680",
"journal-title": "Am J Pathol",
"key": "R7-20210618",
"volume": "190",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.4783",
"article-title": "Treatment of 5 critically ill patients with COVID-19 with convalescent plasma.",
"author": "Shen",
"doi-asserted-by": "crossref",
"first-page": "1582",
"journal-title": "JAMA",
"key": "R8-20210618",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1002/jmv.25882",
"article-title": "Treatment with convalescent plasma for COVID-19 patients in Wuhan, China.",
"author": "Ye",
"doi-asserted-by": "crossref",
"first-page": "1890",
"journal-title": "J Med Virol",
"key": "R9-20210618",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-1088-9",
"article-title": "Convalescent plasma treatment of severe COVID-19: A propensity score-matched control study.",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "1708",
"journal-title": "Nat Med",
"key": "R10-20210618",
"volume": "26",
"year": "2020"
},
{
"article-title": "Convalescent plasma for patients with severe COVID-19: A matched cohort study.",
"author": "Rogers",
"journal-title": "Clin Infect Dis",
"key": "R11-20210618",
"year": "2020 Oct 10"
},
{
"DOI": "10.1016/j.ajpath.2020.08.001",
"article-title": "Treatment of COVID-19 patients with convalescent plasma reveals a signal of significantly decreased mortality.",
"author": "Salazar",
"doi-asserted-by": "crossref",
"first-page": "2290",
"journal-title": "Am J Pathol",
"key": "R12-20210618",
"volume": "190",
"year": "2020"
},
{
"DOI": "10.1172/JCI140200",
"article-title": "Early safety indicators of COVID-19 convalescent plasma in 5000 patients.",
"author": "Joyner",
"doi-asserted-by": "crossref",
"first-page": "4791",
"journal-title": "J Clin Invest",
"key": "R14-20210618",
"volume": "130",
"year": "2020"
},
{
"DOI": "10.1016/j.mayocp.2020.06.028",
"article-title": "Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients.",
"author": "Joyner",
"doi-asserted-by": "crossref",
"first-page": "1888",
"journal-title": "Mayo Clin Proc",
"key": "R15-20210618",
"volume": "95",
"year": "2020"
},
{
"article-title": "Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: Initial three-month experience.",
"author": "Joyner",
"journal-title": "medRxiv",
"key": "R16-20210618"
},
{
"DOI": "10.1136/bmj.m3939",
"article-title": "Convalescent plasma in the management of moderate Covid-19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID Trial).",
"author": "Agarwal",
"doi-asserted-by": "crossref",
"first-page": "m3939",
"journal-title": "BMJ",
"key": "R18-20210618",
"volume": "371",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.10044",
"article-title": "Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: A randomized clinical trial.",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "460",
"journal-title": "JAMA",
"key": "R19-20210618",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2031304",
"article-title": "A randomized trial of convalescent plasma in COVID-19 severe pneumonia.",
"author": "Simonovich",
"doi-asserted-by": "crossref",
"first-page": "619",
"journal-title": "N Engl J Med",
"key": "R20-20210618",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2033700",
"article-title": "Early high-titer plasma therapy to prevent severe COVID-19 in older adults.",
"author": "Libster",
"doi-asserted-by": "crossref",
"first-page": "610",
"journal-title": "N Engl J Med",
"key": "R21-20210618",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1111/trf.16293",
"article-title": "Impact of serological and PCR testing requirements on the selection of COVID-19 convalescent plasma donors.",
"author": "Carter",
"doi-asserted-by": "crossref",
"journal-title": "Transfusion",
"key": "R22-20210618",
"year": "2021 Feb 8"
},
{
"DOI": "10.1111/trf.16283",
"article-title": "Neutralizing activity to SARS-CoV-2 of convalescent and control plasma used in a randomized controlled trial.",
"author": "Freedenberg",
"doi-asserted-by": "crossref",
"journal-title": "Transfusion",
"key": "R23-20210618",
"year": "2021 Jan 15"
},
{
"DOI": "10.1016/S2213-2600(19)30199-7",
"article-title": "Anti-influenza immune plasma for the treatment of patients with severe influenza A: A randomised, double-blind, phase 3 trial.",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "941",
"journal-title": "Lancet Respir Med",
"key": "R24-20210618",
"volume": "7",
"year": "2019"
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.lww.com/10.1097/CCM.0000000000005066"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Critical Care and Intensive Care Medicine"
],
"subtitle": [
"A Double-Blind Randomized Trial"
],
"title": "Severe Acute Respiratory Syndrome Coronavirus 2 Convalescent Plasma Versus Standard Plasma in Coronavirus Disease 2019 Infected Hospitalized Patients in New York",
"type": "journal-article",
"volume": "Publish Ahead of Print"
}