Ramipril for the Treatment of COVID-19: RAMIC, a Randomized, Double-Blind, Placebo-Controlled Clinical Trial
et al., Advances in Therapy, doi:10.1007/s12325-023-02618-7, Aug 2023
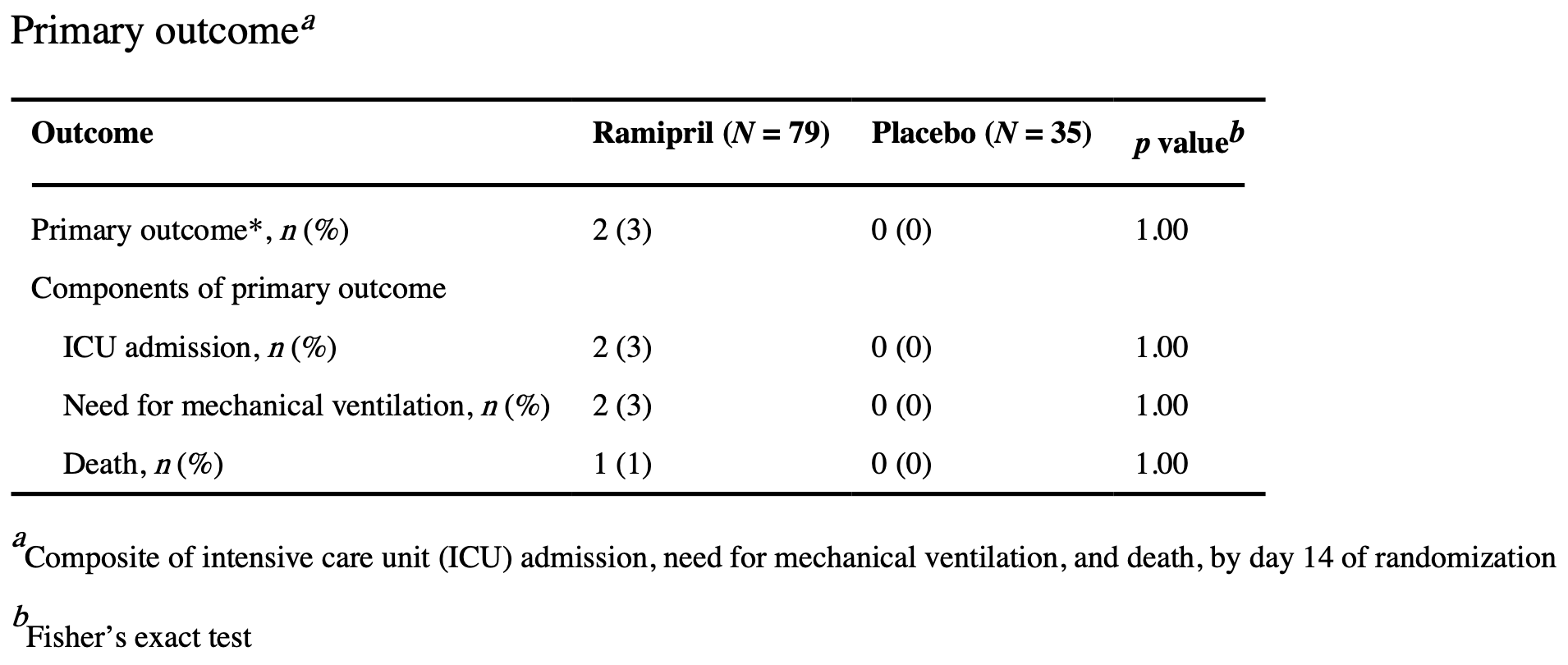
RCT 114 non-critically ill COVID-19 patients in the USA showing no significant differences with ramipril treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 144.3% higher, RR 2.44, p = 1.00, treatment 1 of 79 (1.3%), control 0 of 35 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), day 14.
|
|
risk of mechanical ventilation, 288.6% higher, RR 3.89, p = 1.00, treatment 2 of 79 (2.5%), control 0 of 35 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), day 14.
|
|
risk of ICU admission, 288.6% higher, RR 3.89, p = 1.00, treatment 2 of 79 (2.5%), control 0 of 35 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), day 14.
|
|
risk of no hospital discharge, 144.3% higher, RR 2.44, p = 1.00, treatment 1 of 79 (1.3%), control 0 of 35 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), day 14.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Huang et al., 24 Aug 2023, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 29 authors.
Ramipril is an oral small molecule angiotensin-converting enzyme (ACE) inhibitor that blocks the renin-angiotensin-aldosterone system (RAAS).
Abstract: HHS Public Access
Author manuscript
Author Manuscript
Adv Ther. Author manuscript; available in PMC 2024 May 01.
Published in final edited form as:
Adv Ther. 2023 November ; 40(11): 4805–4816. doi:10.1007/s12325-023-02618-7.
Ramipril for the Treatment of COVID-19: RAMIC, a Randomized,
Double-Blind, Placebo-Controlled Clinical Trial
Daniel Q. Huang,
NAFLD Research Center, Division of Gastroenterology, University of California at San Diego, La
Jolla, CA, USA
Author Manuscript
Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore,
Singapore, Singapore
Division of Gastroenterology and Hepatology, Department of Medicine, National University Health
System, Singapore, Singapore
Veeral Ajmera,
NAFLD Research Center, Division of Gastroenterology, University of California at San Diego, La
Jolla, CA, USA
Division of Gastroenterology, Department of Medicine, University of California San Diego, La
Jolla, CA, USA
Author Manuscript
Christian Tomaszewski,
Department of Emergency Medicine, University of California, San Diego and the El Centro
Regional, Medical Center, San Diego, CA, USA
Andrew LaFree,
Department of Emergency Medicine, University of California, San Diego and the El Centro
Regional, Medical Center, San Diego, CA, USA
Ricki Bettencourt,
NAFLD Research Center, Division of Gastroenterology, University of California at San Diego, La
Jolla, CA, USA
Wesley K. Thompson,
Author Manuscript
roloomba@ucsd.edu .
Author Contributions. Study design: Rohit Loomba, Veeral Ajmera, Daniel Huang. Data acquisition: Veeral Ajmera, Daniel Huang,
Christian Tomaszewski, Andrew LaFree, Jinho Jung, Maral Amangurbanova, Michelle S. Harkins, Wesley K Thompson, Davey M
Smith, Atul Malhotra, Ravindra L Mehta, Vaishal Tolia, Jeffery Yin, Paul A Insel, Stone Leachman, Summer Collier, Lissa Richards,
Kristin Woods, Archana Bhatt, Xinlian Zhang, Oana M Penciu, Stuart Zarich, Tamrat Retta, J Pedro Teixeira, Brian Chinnock,
Netanya S Utay, Jordan Lake, Rohit Loomba. Data analysis: Ricki Bettencourt, Daniel Huang, Rohit Loomba. Data interpretation and
review/revision of the manuscript: All authors. Study concept and study supervision: Rohit Loomba. All authors approved the final
draft of the manuscript as well as the authorship list.
Daniel Q. Huang and Veeral Ajmera share co-first authorship.
Compliance with Ethics Guidelines. This study was approved by the institutional review board for all sites (Advarra IRB approved
Apr 2020, A Randomized, Double-blind, Placebo Controlled Trial to Evaluate the Efficacy of Ramipril to Prevent ICU Admission,
Mechanical Ventilation or Death in Persons with COVID-19 [Pro00043364]). Consent was obtained from each patient, and was
received either in-person, or by videoconference or telephone, which occurred in the presence of a co-signing witness. All authors
agree with the manuscript and consent for its publication.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/
s12325-023-02618-7.
Huang et al.
Page 2
Author Manuscript
Division of Biostatistics and Bioinformatics, Department of Family Medicine and Public Health,
University of California San Diego, La Jolla, CA, USA
Davey M. Smith,
Division of Infectious Diseases and Global Public Health, Department of Medicine, University of
California San Diego, La Jolla, CA, USA
Veteran Affairs Medical Center, San Diego, CA, USA
Atul Malhotra,
Division of Pulmonary, Critical Care and Sleep..
DOI record:
{
"DOI": "10.1007/s12325-023-02618-7",
"ISSN": [
"0741-238X",
"1865-8652"
],
"URL": "http://dx.doi.org/10.1007/s12325-023-02618-7",
"alternative-id": [
"2618"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "23 May 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "14 July 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "24 August 2023"
}
],
"author": [
{
"affiliation": [],
"family": "Huang",
"given": "Daniel Q.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ajmera",
"given": "Veeral",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tomaszewski",
"given": "Christian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "LaFree",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bettencourt",
"given": "Ricki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thompson",
"given": "Wesley K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smith",
"given": "Davey M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Malhotra",
"given": "Atul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mehta",
"given": "Ravindra L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tolia",
"given": "Vaishal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yin",
"given": "Jeffrey",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Insel",
"given": "Paul A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leachman",
"given": "Stone",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jung",
"given": "Jinho",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Collier",
"given": "Summer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Richards",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Woods",
"given": "Kristin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Amangurbanova",
"given": "Maral",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bhatt",
"given": "Archana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Xinlian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Penciu",
"given": "Oana M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zarich",
"given": "Stuart",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Retta",
"given": "Tamrat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harkins",
"given": "Michelle S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Teixeira",
"given": "J. Pedro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chinnock",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Utay",
"given": "Netanya S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lake",
"given": "Jordan E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Loomba",
"given": "Rohit",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct04366050",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": "Advances in Therapy",
"container-title-short": "Adv Ther",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2023,
8,
24
]
],
"date-time": "2023-08-24T11:03:07Z",
"timestamp": 1692874987000
},
"deposited": {
"date-parts": [
[
2023,
10,
11
]
],
"date-time": "2023-10-11T10:36:53Z",
"timestamp": 1697020613000
},
"funder": [
{
"DOI": "10.13039/100004319",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100004319",
"id-type": "DOI"
}
],
"name": "Pfizer"
}
],
"indexed": {
"date-parts": [
[
2025,
11,
5
]
],
"date-time": "2025-11-05T21:17:12Z",
"timestamp": 1762377432565,
"version": "3.37.3"
},
"is-referenced-by-count": 4,
"issue": "11",
"issued": {
"date-parts": [
[
2023,
8,
24
]
]
},
"journal-issue": {
"issue": "11",
"published-print": {
"date-parts": [
[
2023,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.springernature.com/gp/researchers/text-and-data-mining",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
8,
24
]
],
"date-time": "2023-08-24T00:00:00Z",
"timestamp": 1692835200000
}
},
{
"URL": "https://www.springernature.com/gp/researchers/text-and-data-mining",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
8,
24
]
],
"date-time": "2023-08-24T00:00:00Z",
"timestamp": 1692835200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s12325-023-02618-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s12325-023-02618-7/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s12325-023-02618-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"page": "4805-4816",
"prefix": "10.1007",
"published": {
"date-parts": [
[
2023,
8,
24
]
]
},
"published-online": {
"date-parts": [
[
2023,
8,
24
]
]
},
"published-print": {
"date-parts": [
[
2023,
11
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "2618_CR1",
"unstructured": "WHO. WHO coronavirus (COVID-19) dashboard. https://covid19.who.int Accessed Feb 1, 2022."
},
{
"DOI": "10.1001/jama.2020.6775",
"author": "S Richardson",
"doi-asserted-by": "publisher",
"first-page": "2052",
"issue": "20",
"journal-title": "JAMA",
"key": "2618_CR2",
"unstructured": "Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–9.",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.12839",
"author": "WJ Wiersinga",
"doi-asserted-by": "publisher",
"first-page": "782",
"issue": "8",
"journal-title": "JAMA",
"key": "2618_CR3",
"unstructured": "Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–93.",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1002/jmv.26424",
"author": "J Li",
"doi-asserted-by": "publisher",
"first-page": "1449",
"issue": "3",
"journal-title": "J Med Virol",
"key": "2618_CR4",
"unstructured": "Li J, Huang DQ, Zou B, et al. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2020;93(3):1449–58.",
"volume": "93",
"year": "2020"
},
{
"DOI": "10.1161/CIRCULATIONAHA.104.510461",
"author": "CM Ferrario",
"doi-asserted-by": "publisher",
"first-page": "2605",
"issue": "20",
"journal-title": "Circulation",
"key": "2618_CR5",
"unstructured": "Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–10.",
"volume": "111",
"year": "2005"
},
{
"DOI": "10.1097/HJH.0b013e32834f04b6",
"author": "A Soro-Paavonen",
"doi-asserted-by": "publisher",
"first-page": "375",
"issue": "2",
"journal-title": "J Hypertens",
"key": "2618_CR6",
"unstructured": "Soro-Paavonen A, Gordin D, Forsblom C, et al. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J Hypertens. 2012;30(2):375–83.",
"volume": "30",
"year": "2012"
},
{
"DOI": "10.1038/s41586-020-2179-y",
"author": "J Shang",
"doi-asserted-by": "publisher",
"first-page": "221",
"issue": "7807",
"journal-title": "Nature",
"key": "2618_CR7",
"unstructured": "Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–4.",
"volume": "581",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2008975",
"author": "HR Reynolds",
"doi-asserted-by": "publisher",
"first-page": "2441",
"issue": "25",
"journal-title": "N Engl J Med",
"key": "2618_CR8",
"unstructured": "Reynolds HR, Adhikari S, Pulgarin C, et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382(25):2441–8.",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2006923",
"author": "G Mancia",
"doi-asserted-by": "publisher",
"first-page": "2431",
"issue": "25",
"journal-title": "N Engl J Med",
"key": "2618_CR9",
"unstructured": "Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382(25):2431–40.",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.11301",
"author": "EL Fosbøl",
"doi-asserted-by": "publisher",
"first-page": "168",
"issue": "2",
"journal-title": "JAMA",
"key": "2618_CR10",
"unstructured": "Fosbøl EL, Butt JH, Østergaard L, et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA. 2020;324(2):168–77.",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1161/CIRCRESAHA.120.317134",
"author": "P Zhang",
"doi-asserted-by": "publisher",
"first-page": "1671",
"issue": "12",
"journal-title": "Circ Res",
"key": "2618_CR11",
"unstructured": "Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126(12):1671–81.",
"volume": "126",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2021.3594",
"author": "R Baral",
"doi-asserted-by": "publisher",
"issue": "3",
"journal-title": "JAMA Netw Open",
"key": "2618_CR12",
"unstructured": "Baral R, Tsampasian V, Debski M, et al. Association between renin-angiotensin-aldosterone system inhibitors and clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(3):e213594.",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1161/JAHA.120.018086",
"author": "R Khera",
"doi-asserted-by": "publisher",
"issue": "13",
"journal-title": "J Am Heart Assoc",
"key": "2618_CR13",
"unstructured": "Khera R, Clark C, Lu Y, et al. Association of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers with the risk of hospitalization and death in hypertensive patients with COVID-19. J Am Heart Assoc. 2021;10(13):e018086.",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.7326/M20-1515",
"author": "K Mackey",
"doi-asserted-by": "publisher",
"first-page": "195",
"issue": "3",
"journal-title": "Ann Intern Med",
"key": "2618_CR14",
"unstructured": "Mackey K, King VJ, Gurley S, et al. Risks and impact of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers on SARS-CoV-2 infection in adults: a living systematic review. Ann Intern Med. 2020;173(3):195–203.",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.1007/s00134-020-05985-9",
"author": "H Zhang",
"doi-asserted-by": "publisher",
"first-page": "586",
"issue": "4",
"journal-title": "Intensive Care Med",
"key": "2618_CR15",
"unstructured": "Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–90.",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1038/nature03712",
"author": "Y Imai",
"doi-asserted-by": "publisher",
"first-page": "112",
"issue": "7047",
"journal-title": "Nature",
"key": "2618_CR16",
"unstructured": "Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–6.",
"volume": "436",
"year": "2005"
},
{
"DOI": "10.1073/pnas.0711241105",
"author": "S Haga",
"doi-asserted-by": "publisher",
"first-page": "7809",
"issue": "22",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "2618_CR17",
"unstructured": "Haga S, Yamamoto N, Nakai-Murakami C, et al. Modulation of TNF-α-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc Natl Acad Sci U S A. 2008;105(22):7809–14.",
"volume": "105",
"year": "2008"
},
{
"DOI": "10.1111/bph.15082",
"author": "K Sriram",
"doi-asserted-by": "publisher",
"first-page": "4825",
"issue": "21",
"journal-title": "Br J Pharmacol",
"key": "2618_CR18",
"unstructured": "Sriram K, Insel PA. A hypothesis for pathobiology and treatment of COVID-19: the centrality of ACE1/ACE2 imbalance. Br J Pharmacol. 2020;177(21):4825–44.",
"volume": "177",
"year": "2020"
},
{
"DOI": "10.1038/nm1267",
"author": "K Kuba",
"doi-asserted-by": "publisher",
"first-page": "875",
"issue": "8",
"journal-title": "Nat Med",
"key": "2618_CR19",
"unstructured": "Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–9.",
"volume": "11",
"year": "2005"
},
{
"DOI": "10.1016/j.cct.2021.106330",
"author": "V Ajmera",
"doi-asserted-by": "publisher",
"journal-title": "Contemp Clin Trials",
"key": "2618_CR20",
"unstructured": "Ajmera V, Thompson WK, Smith DM, et al. RAMIC: design of a randomized, double-blind, placebo-controlled trial to evaluate the efficacy of ramipril in patients with COVID-19. Contemp Clin Trials. 2021;103:106330.",
"volume": "103",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.24415",
"author": "The Medical Letter",
"doi-asserted-by": "publisher",
"first-page": "880",
"issue": "9",
"journal-title": "JAMA",
"key": "2618_CR21",
"unstructured": "The Medical Letter. An EUA for bamlanivimab–a monoclonal antibody for COVID-19. JAMA. 2021;325(9):880–1.",
"volume": "325",
"year": "2021"
},
{
"key": "2618_CR22",
"unstructured": "COVID-19 Update: FDA broadens emergency use authorization for Veklury (remdesivir) to include all hospitalized patients for treatment of COVID-19. Accessed Feb 3, 2022. https://www.fda.gov/news-events/press-announcements/covid-19-update-fda-broadens-emergency-use-authorization-veklury-remdesivir-include-all-hospitalized. 2020."
},
{
"DOI": "10.1136/bmj.m1996",
"author": "MG Argenziano",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "2618_CR23",
"unstructured": "Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369: m1996.",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30558-0",
"author": "JB Cohen",
"doi-asserted-by": "publisher",
"first-page": "275",
"issue": "3",
"journal-title": "Lancet Respir Med",
"key": "2618_CR24",
"unstructured": "Cohen JB, Hanff TC, William P, et al. Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir Med. 2021;9(3):275–84.",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2021.100957",
"author": "MA Puskarich",
"doi-asserted-by": "publisher",
"journal-title": "EClinicalMedicine",
"key": "2618_CR25",
"unstructured": "Puskarich MA, Cummins NW, Ingraham NE, et al. A multi-center phase II randomized clinical trial of losartan on symptomatic outpatients with COVID-19. EClinicalMedicine. 2021;37:100957.",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1001/jama.2023.4480",
"author": "PR Lawler",
"doi-asserted-by": "publisher",
"first-page": "1183",
"issue": "14",
"journal-title": "JAMA",
"key": "2618_CR26",
"unstructured": "Writing Committee for the REMAP-CAP Investigators, Lawler PR, Derde LPG, et al. Effect of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker initiation on organ support-free days in patients hospitalized with COVID-19: a randomized clinical trial. JAMA. 2023;329(14):1183–96.",
"volume": "329",
"year": "2023"
}
],
"reference-count": 26,
"references-count": 26,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s12325-023-02618-7"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Ramipril for the Treatment of COVID-19: RAMIC, a Randomized, Double-Blind, Placebo-Controlled Clinical Trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "40"
}