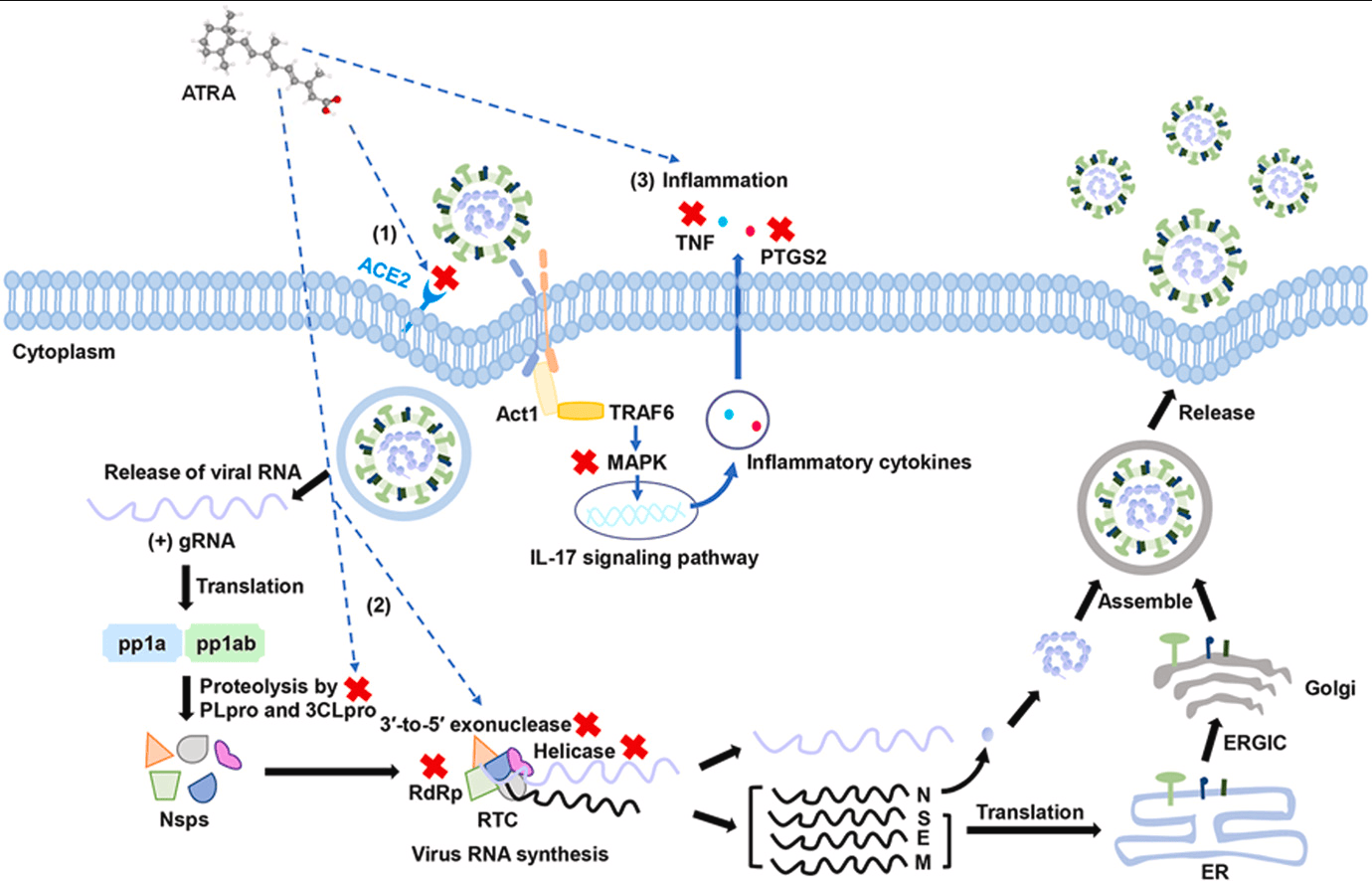
All-trans retinoic acid acts as a dual-purpose inhibitor of SARS-CoV-2 infection and inflammation
et al., Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2024.107942, Jan 2024
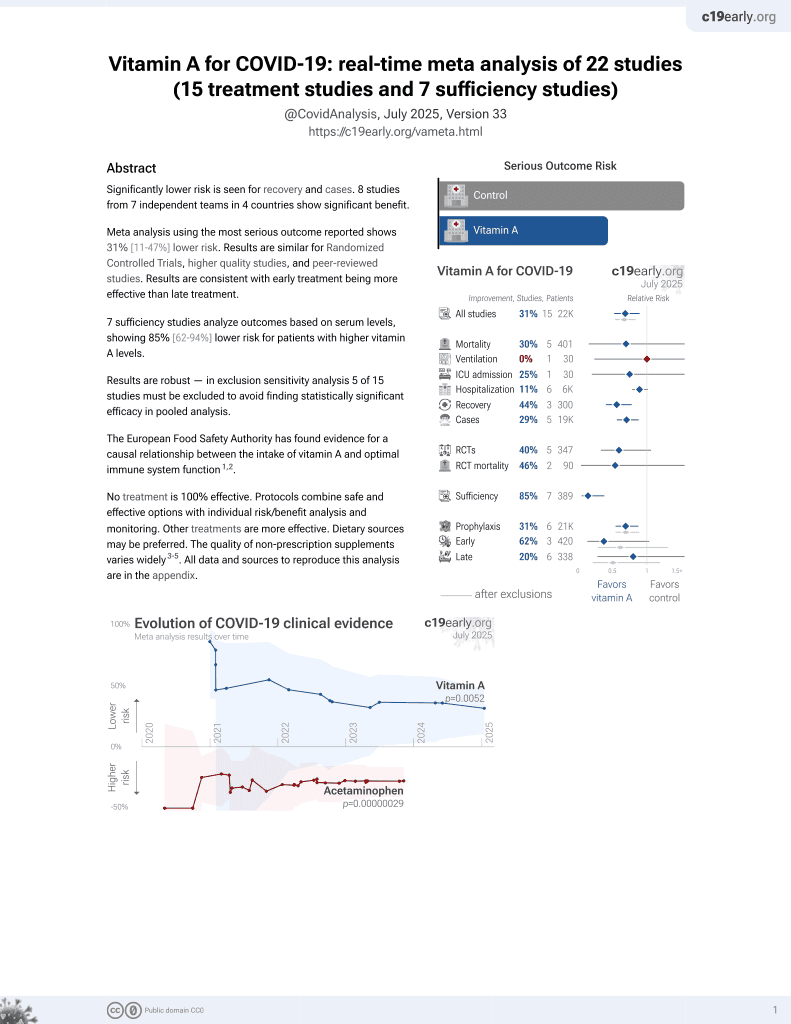
Vitamin A for COVID-19
49th treatment shown to reduce risk in
May 2023, now with p = 0.004 from 14 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
In vitro and in silico study showing that all-trans retinoic acid (an active metabolite of vitamin A) inhibits the entry and replication of SARS-CoV-2 by binding to ACE2 / 3CLpro / RdRp / helicase / 3′-to-5′ exonuclease, and reduces excessive inflammation induced by SARS-CoV-2.
11 preclinical studies support the efficacy of vitamin A for COVID-19:
Vitamin A has been identified by the European Food Safety Authority (EFSA) as having sufficient evidence for a causal relationship between intake and optimal immune system function11-13.
Vitamin A has potent antiviral activity against SARS-CoV-2 in both human cell lines and human organoids of the lower respiratory tract (active metabolite all-trans retinoic acid, ATRA)8, is predicted to bind critical host and viral proteins for SARS-CoV-2 and may compensate for gene expression changes related to SARS-CoV-22-4, may be beneficial for COVID-19 via antiviral, anti-inflammatory, and immunomodulatory effects according to network pharmacology analysis5, reduces barrier compromise caused by TNF-α in Calu-3 cells7, inhibits mouse coronavirus replication10, may stimulate innate immunity by activating interferon responses in an IRF3-dependent manner (ATRA)10, may reduce excessive inflammation induced by SARS-CoV-22, shows SARS-CoV-2 antiviral activity In Vitro2,6,9 , is effective against multiple SARS-CoV-2 variants in Calu-3 cells9, and inhibits the entry and replication of SARS-CoV-2 via binding to ACE2 / 3CLpro / RdRp / helicase / 3'-to-5' exonuclease2.
1.
Voloudakis et al., A genetically based computational drug repurposing framework for rapid identification of candidate compounds: application to COVID-19, medRxiv, doi:10.1101/2025.01.10.25320348.
2.
Huang et al., All-trans retinoic acid acts as a dual-purpose inhibitor of SARS-CoV-2 infection and inflammation, Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2024.107942.
3.
Chakraborty et al., In-silico screening and in-vitro assay show the antiviral effect of Indomethacin against SARS-CoV-2, Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2022.105788.
4.
Pandya et al., Unravelling Vitamin B12 as a potential inhibitor against SARS-CoV-2: A computational approach, Informatics in Medicine Unlocked, doi:10.1016/j.imu.2022.100951.
5.
Li et al., Revealing the targets and mechanisms of vitamin A in the treatment of COVID-19, Aging, doi:10.18632/aging.103888.
6.
Moatasim et al., Potent Antiviral Activity of Vitamin B12 against Severe Acute Respiratory Syndrome Coronavirus 2, Middle East Respiratory Syndrome Coronavirus, and Human Coronavirus 229E, Microorganisms, doi:10.3390/microorganisms11112777.
7.
DiGuilio et al., The multiphasic TNF-α-induced compromise of Calu-3 airway epithelial barrier function, Experimental Lung Research, doi:10.1080/01902148.2023.2193637.
8.
Tong et al., A Retinol Derivative Inhibits SARS-CoV-2 Infection by Interrupting Spike-Mediated Cellular Entry, mBio, doi:10.1128/mbio.01485-22.
9.
Morita et al., All-Trans Retinoic Acid Exhibits Antiviral Effect against SARS-CoV-2 by Inhibiting 3CLpro Activity, Viruses, doi:10.3390/v13081669.
10.
Franco et al., Retinoic Acid-Mediated Inhibition of Mouse Coronavirus Replication Is Dependent on IRF3 and CaMKK, Viruses, doi:10.3390/v16010140.
11.
Galmés et al., Suboptimal Consumption of Relevant Immune System Micronutrients Is Associated with a Worse Impact of COVID-19 in Spanish Populations, Nutrients, doi:10.3390/nu14112254.
12.
Galmés (B) et al., Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework, Nutrients, doi:10.3390/nu12092738.
13.
EFSA, Scientific Opinion on the substantiation of health claims related to vitamin A and cell differentiation (ID 14), function of the immune system (ID 14), maintenance of skin and mucous membranes (ID 15, 17), maintenance of vision (ID 16), maintenance of bone (ID 13, 17), maintenance of teeth (ID 13, 17), maintenance of hair (ID 17), maintenance of nails (ID 17), metabolism of iron (ID 206), and protection of DNA, proteins and lipids from oxidative damage (ID 209) pursuant to Article 13(1) of Regulation (EC) No 1924/2006, EFSA Journal, doi:10.2903/j.efsa.2009.1221.
Huang et al., 3 Jan 2024, peer-reviewed, 7 authors.
Contact: leijian@scu.edu.cn, hinchu@hku.hk, yangsy@scu.edu.cn.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
A new generation Mpro inhibitor with potent activity against SARS-CoV-2 Omicron variants
Signal Transduction and Targeted Therapy, doi:10.1038/s41392-023-01392-w
Emerging SARS-CoV-2 variants, particularly the Omicron variant and its sublineages, continually threaten the global public health. Small molecule antivirals are an effective treatment strategy to fight against the virus. However, the first-generation antivirals either show limited clinical efficacy and/or have some defects in pharmacokinetic (PK) properties. Moreover, with increased use of these drugs across the globe, they face great pressure of drug resistance. We herein present the discovery and characterization of a new generation antiviral drug candidate (SY110), which is a potent and selective inhibitor of SARS-CoV-2 main protease (M pro ). This compound displayed potent in vitro antiviral activity against not only the predominant SARS-CoV-2 Omicron sublineage BA.5, but also other highly pathogenic human coronaviruses including SARS-CoV-1 and MERS-CoV. In the Omicron-infected K18-hACE2 mouse model, oral treatment with SY110 significantly lowered the viral burdens in lung and alleviated the virus-induced pathology. Importantly, SY110 possesses favorable PK properties with high oral drug exposure and oral bioavailability, and also an outstanding safety profile. Furthermore, SY110 exhibited sensitivity to several drug-resistance M pro mutations. Collectively, this investigation provides a promising new drug candidate against Omicron and other variants of SARS-CoV-2.
AUTHOR CONTRIBUTIONS S.Y., H.C., and J.L. conceived research; and C.H. performed the drug design; C.H., A.X., and Z.F. with the assistance of Q.H., J.Y., B.Q., N.G., S.Z., R.M., J.Z., S.Z., J.N., H.X., and F.W. performed the chemical synthesis; R.Z., L.X., J.Q., and X.Z. performed gene expression, protein purification and crystallization experiments; R.Z., L.X., and J.L. determined the crystal structures; J.Q. and Y.L. performed high-throughput screening, enzymatic activity and inhibition assays, IC 50 measurements, DSF assays, cytotoxicity assays and in vivo toxicity assays; H.S., Y.H., C.Y., and B.H. performed in vitro and in vivo antiviral assays; S.Y., J.L., and H.C. with the assistance of C.H., H.S., J.Q., and R.Z. wrote and revised the manuscript. All authors have read and approved the article.
ADDITIONAL INFORMATION Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41392-023-01392-w. Competing interests: All authors declared no competing interests. S.Y. is the member of editorial board, he has not been involved in the process of the manuscript handling.
References
Afonine, Towards automated crystallographic structure refnement with phenix, refne. Acta Crystallogr. D. Biol. Crystallogr
Anand, Ziebuhr, Wadhwani, Mesters, Hilgenfeld, Coronavirus main proteinase (3CL pro ) structure: basis for design of anti-SARS drugs, Science
Andrews, Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant, N. Engl. J. Med
Bricogne, None, BUSTER version
Cao, 4 and BA.5 escape antibodies elicited by Omicron infection, Nature
Chan, Virological features and pathogenicity of SARS-CoV-2 Omicron BA.2, Cell Rep. Med
Chu, Host and viral determinants for efficient SARS-CoV-2 infection of the human lung, Nat. Commun
Dai, Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease, Science
Emsley, Features and development of Coot, Acta Crystallogr. D. Biol. Crystallogr
Evans, How good are my data and what is the resolution?, Acta Crystallogr. D. Biol. Crystallogr
Fu, Both Boceprevir and GC376 efficaciously inhibit SARS-CoV-2 by targeting its main protease, Nat. Commun
Garcia-Beltran, mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant, Cell
Halfmann, SARS-CoV-2 Omicron virus causes attenuated disease in mice and hamsters, Nature
Han, Structure-based optimization of ML300-derived, noncovalent inhibitors targeting the severe acute respiratory syndrome coronavirus 3CL protease (SARS-CoV-2 3CL pro ), J. Med. Chem
Hegyi, Ziebuhr, Conservation of substrate specificities among coronavirus main proteases, J. Gen. Virol
Heilmann, SARS-CoV-2 3CL pro mutations selected in a VSV-based system confer resistance to nirmatrelvir, ensitrelvir, and GC376, Sci. Transl. Med
Hilgenfeld, From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design, FEBS J
Hu, Naturally occurring mutations of SARS-CoV-2 main protease confer drug resistance to nirmatrelvir, bioRxiv, doi:10.1101/2022.06.28.497978
Hu, Spike mutations contributing to the altered entry preference of SARS-CoV-2 Omicron BA.1 and BA.2, Emerg. Microbes Infect
Iketani, Antibody evasion properties of SARS-CoV-2 Omicron sublineages, Nature
Iketani, Multiple pathways for SARS-CoV-2 resistance to nirmatrelvir, Nature
Jin, Structural basis for the inhibition of SARS-CoV-2 main protease by antineoplastic drug carmofur, Nat. Struct. Mol. Biol
Jochmans, The substitutions L50F, E166A and L167F in SARS-CoV-2 3CL pro are selected by a protease inhibitor in vitro and confer resistance to nirmatrelvir, mBio
Kabsch, Xds, None, Acta Crystallogr. D. Biol. Crystallogr
Khailany, Safdar, Ozaslan, Genomic characterization of a novel SARS-CoV-2, Gene Rep
Liu, Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2, Nature
Lopez Bernal, Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant, N. Engl. J. Med
Lu, Su, Yang, Jiang, Antivirals with common targets against highly pathogenic viruses, Cell
Mannar, SARS-CoV-2 Omicron variant: antibody evasion and cryo-EM structure of spike protein-ACE2 complex, Science
Moghadasi, Transmissible SARS-CoV-2 variants with resistance to clinical protease inhibitors, bioRxiv, doi:10.1101/2022.08.07.503099
Mohapatra, Twin of Omicron and Delta variants triggering a tsunami wave of ever high surges in COVID-19 cases: a challenging global threat with a special focus on the Indian subcontinent, J. Med. Virol
Noske, Structural basis of nirmatrelvir and ensitrelvir resistance profiles against SARS-CoV-2 Main Protease naturally occurring polymorphisms, J. Biol. Chem
Owen, An oral SARS-CoV-2 M pro inhibitor clinical candidate for the treatment of COVID-19, Science
Planas, Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization, Nature
Qiao, SARS-CoV-2 M pro inhibitors with antiviral activity in a transgenic mouse model, Science
Quan, An orally available M pro inhibitor is effective against wild-type SARS-CoV-2 and variants including Omicron, Nat. Microbiol
Rathnayake, 3C-like protease inhibitors block coronavirus replication in vitro and improve survival in MERS-CoV-infected mice, Sci. Transl. Med
Saad-Roy, Immune life history, vaccination, and the dynamics of SARS-CoV-2 over the next 5 years, Science
Sacco, Structure and inhibition of the SARS-CoV-2 main protease reveal strategy for developing dual inhibitors against M pro and cathepsin, L. Sci. Adv
Sasi, Predicting antiviral resistance mutations in SARS-CoV-2 main protease with computational and experimental screening, Biochemistry
Shuai, Attenuated replication and pathogenicity of SARS-CoV-2 B.1.1.529 Omicron, Nature
Shuai, Emerging SARS-CoV-2 variants expand species tropism to murines, EBioMedicine
Suzuki, Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant, Nature
Tuekprakhon, Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum, Cell
Unoh, Discovery of S-217622, a noncovalent oral SARS-CoV-2 3CL protease inhibitor clinical candidate for treating COVID-19, J. Med. Chem
Wahl, SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801, Nature
Williamson, Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2, Nature
Wu, A new coronavirus associated with human respiratory disease in China, Nature
Wu, Yin, Jiang, Xu, Structure genomics of SARS-CoV-2 and its Omicron variant: drug design templates for COVID-19, Acta Pharmacol. Sin
Xie, Design and development of an oral remdesivir derivative VV116 against SARS-CoV-2, Cell Res
Yan, Florian, Why remdesivir failed: preclinical assumptions overestimate the clinical efficacy of remdesivir for COVID-19 and Ebola, Antimicrob. Agents Chemother
Zhang, Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients, Signal Transduct. Target Ther
Zhang, Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors, Science
Zhou, A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature
Zhou, Nirmatrelvir resistant SARS-CoV-2 variants with high fitness in vitro, Sci Adv
DOI record:
{
"DOI": "10.1016/j.compbiomed.2024.107942",
"ISSN": [
"0010-4825"
],
"URL": "http://dx.doi.org/10.1016/j.compbiomed.2024.107942",
"alternative-id": [
"S001048252400026X"
],
"article-number": "107942",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "All-trans retinoic acid acts as a dual-purpose inhibitor of SARS-CoV-2 infection and inflammation"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Computers in Biology and Medicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.compbiomed.2024.107942"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Huang",
"given": "Juanjuan",
"sequence": "first"
},
{
"affiliation": [],
"family": "Fang",
"given": "Yabo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wu",
"given": "Runze",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xia",
"given": "Tingzheng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Xuan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9055-7627",
"affiliation": [],
"authenticated-orcid": false,
"family": "Jia",
"given": "Jiwei",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9326-3755",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wang",
"given": "Guoqing",
"sequence": "additional"
}
],
"container-title": "Computers in Biology and Medicine",
"container-title-short": "Computers in Biology and Medicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
1,
4
]
],
"date-time": "2024-01-04T00:27:15Z",
"timestamp": 1704328035000
},
"deposited": {
"date-parts": [
[
2024,
2,
2
]
],
"date-time": "2024-02-02T14:57:10Z",
"timestamp": 1706885830000
},
"indexed": {
"date-parts": [
[
2024,
2,
3
]
],
"date-time": "2024-02-03T00:22:53Z",
"timestamp": 1706919773016
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
2
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
1
]
],
"date-time": "2024-02-01T00:00:00Z",
"timestamp": 1706745600000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
1
]
],
"date-time": "2024-02-01T00:00:00Z",
"timestamp": 1706745600000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
1
]
],
"date-time": "2024-02-01T00:00:00Z",
"timestamp": 1706745600000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
1
]
],
"date-time": "2024-02-01T00:00:00Z",
"timestamp": 1706745600000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
1
]
],
"date-time": "2024-02-01T00:00:00Z",
"timestamp": 1706745600000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
1
]
],
"date-time": "2024-02-01T00:00:00Z",
"timestamp": 1706745600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S001048252400026X?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S001048252400026X?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "107942",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
2
]
]
},
"published-print": {
"date-parts": [
[
2024,
2
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"article-title": "A novel coronavirus from patients with pneumonia in China, 2019",
"author": "Zhu",
"doi-asserted-by": "crossref",
"first-page": "727",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.compbiomed.2024.107942_bib1",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/j.celrep.2021.110218",
"article-title": "The SARS-CoV-2 Lambda variant exhibits enhanced infectivity and immune resistance",
"author": "Kimura",
"doi-asserted-by": "crossref",
"journal-title": "Cell Rep.",
"key": "10.1016/j.compbiomed.2024.107942_bib2",
"volume": "38",
"year": "2022"
},
{
"DOI": "10.1038/s41573-023-00672-y",
"article-title": "Therapeutic strategies for COVID-19: progress and lessons learned",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "449",
"journal-title": "Nat. Rev. Drug Discov.",
"key": "10.1016/j.compbiomed.2024.107942_bib3",
"volume": "22",
"year": "2023"
},
{
"DOI": "10.1016/j.cell.2020.03.045",
"article-title": "Structural and functional basis of SARS-CoV-2 entry by using human ACE2",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "894",
"journal-title": "Cell",
"key": "10.1016/j.compbiomed.2024.107942_bib4",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2180-5",
"article-title": "Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor",
"author": "Lan",
"doi-asserted-by": "crossref",
"first-page": "215",
"journal-title": "Nature",
"key": "10.1016/j.compbiomed.2024.107942_bib5",
"volume": "581",
"year": "2020"
},
{
"DOI": "10.1038/s41579-020-00468-6",
"article-title": "Coronavirus biology and replication: implications for SARS-CoV-2",
"author": "V'Kovski",
"doi-asserted-by": "crossref",
"first-page": "155",
"journal-title": "Nat. Rev. Microbiol.",
"key": "10.1016/j.compbiomed.2024.107942_bib6",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1038/s41392-020-0191-1",
"article-title": "Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2",
"author": "Catanzaro",
"doi-asserted-by": "crossref",
"first-page": "84",
"journal-title": "Signal Transduct. Targeted Ther.",
"key": "10.1016/j.compbiomed.2024.107942_bib7",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1080/10408398.2018.1509201",
"article-title": "The success and the challenge of all-trans retinoic acid in the treatment of cancer",
"author": "Ni",
"doi-asserted-by": "crossref",
"first-page": "S71",
"journal-title": "Crit. Rev. Food Sci. Nutr.",
"key": "10.1016/j.compbiomed.2024.107942_bib8",
"volume": "59",
"year": "2019"
},
{
"DOI": "10.1016/S0190-9622(88)70161-9",
"article-title": "Topical tretinoin in the treatment of aging skin",
"author": "Weiss",
"doi-asserted-by": "crossref",
"first-page": "169",
"journal-title": "J. Am. Acad. Dermatol.",
"key": "10.1016/j.compbiomed.2024.107942_bib9",
"volume": "19",
"year": "1988"
},
{
"DOI": "10.1053/j.ajkd.2009.06.012",
"article-title": "Successful treatment with retinoids in patients with lupus nephritis",
"author": "Kinoshita",
"doi-asserted-by": "crossref",
"first-page": "344",
"journal-title": "Am. J. Kidney Dis.",
"key": "10.1016/j.compbiomed.2024.107942_bib10",
"volume": "55",
"year": "2010"
},
{
"DOI": "10.1016/S2352-3026(21)00269-6",
"article-title": "All-trans retinoic acid for treating immune thrombocytopenia: new purpose for an old drug?",
"author": "Gabarin",
"doi-asserted-by": "crossref",
"first-page": "e672",
"journal-title": "Lancet Haematol",
"key": "10.1016/j.compbiomed.2024.107942_bib11",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1111/j.1478-3231.2007.01666.x",
"article-title": "All-trans retinoic acid for treatment of chronic hepatitis C",
"author": "Böcher",
"doi-asserted-by": "crossref",
"first-page": "347",
"journal-title": "Liver Int.",
"key": "10.1016/j.compbiomed.2024.107942_bib12",
"volume": "28",
"year": "2008"
},
{
"DOI": "10.1016/j.antiviral.2008.04.003",
"article-title": "Retinoids inhibit measles virus in vitro via nuclear retinoid receptor signaling pathways",
"author": "Trottier",
"doi-asserted-by": "crossref",
"first-page": "45",
"journal-title": "Antivir. Res.",
"key": "10.1016/j.compbiomed.2024.107942_bib13",
"volume": "80",
"year": "2008"
},
{
"DOI": "10.1039/D3RA00281K",
"article-title": "3DProtDTA: a deep learning model for drug-target affinity prediction based on residue-level protein graphs",
"author": "Voitsitskyi",
"doi-asserted-by": "crossref",
"first-page": "10261",
"journal-title": "RSC Adv.",
"key": "10.1016/j.compbiomed.2024.107942_bib14",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1093/bioinformatics/bty593",
"article-title": "DeepDTA: deep drug-target binding affinity prediction",
"author": "Öztürk",
"doi-asserted-by": "crossref",
"first-page": "i821",
"journal-title": "Bioinformatics",
"key": "10.1016/j.compbiomed.2024.107942_bib15",
"volume": "34",
"year": "2018"
},
{
"DOI": "10.1146/annurev-micro-020518-115759",
"article-title": "Human coronavirus: host-pathogen interaction",
"author": "Fung",
"doi-asserted-by": "crossref",
"first-page": "529",
"journal-title": "Annu. Rev. Microbiol.",
"key": "10.1016/j.compbiomed.2024.107942_bib16",
"volume": "73",
"year": "2019"
},
{
"DOI": "10.1038/s41401-020-0483-6",
"article-title": "Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients",
"author": "Su",
"doi-asserted-by": "crossref",
"first-page": "1167",
"journal-title": "Acta Pharmacol. Sin.",
"key": "10.1016/j.compbiomed.2024.107942_bib17",
"volume": "41",
"year": "2020"
},
{
"DOI": "10.1016/j.cgh.2020.04.002",
"article-title": "Clinical features of COVID-19-related liver functional abnormality",
"author": "Fan",
"doi-asserted-by": "crossref",
"first-page": "1561",
"journal-title": "Clin. Gastroenterol. Hepatol.",
"key": "10.1016/j.compbiomed.2024.107942_bib18",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2020.03.060",
"article-title": "Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19",
"author": "Zhu",
"doi-asserted-by": "crossref",
"first-page": "e21",
"journal-title": "J. Infect.",
"key": "10.1016/j.compbiomed.2024.107942_bib19",
"volume": "81",
"year": "2020"
},
{
"DOI": "10.1093/bib/bbab117",
"article-title": "Deep drug-target binding affinity prediction with multiple attention blocks",
"author": "Zeng",
"doi-asserted-by": "crossref",
"journal-title": "Briefings Bioinf.",
"key": "10.1016/j.compbiomed.2024.107942_bib20",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1016/j.csbj.2020.03.025",
"article-title": "Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model",
"author": "Beck",
"doi-asserted-by": "crossref",
"first-page": "784",
"journal-title": "Comput. Struct. Biotechnol. J.",
"key": "10.1016/j.compbiomed.2024.107942_bib21",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1038/s41392-022-01249-8",
"article-title": "Small molecules in the treatment of COVID-19",
"author": "Lei",
"doi-asserted-by": "crossref",
"first-page": "387",
"journal-title": "Signal Transduct. Targeted Ther.",
"key": "10.1016/j.compbiomed.2024.107942_bib22",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1093/nar/gkq647",
"article-title": "Cooperative translocation enhances the unwinding of duplex DNA by SARS coronavirus helicase nsP13",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "7626",
"journal-title": "Nucleic Acids Res.",
"key": "10.1016/j.compbiomed.2024.107942_bib23",
"volume": "38",
"year": "2010"
},
{
"DOI": "10.1126/science.abi9310",
"article-title": "Structural basis of mismatch recognition by a SARS-CoV-2 proofreading enzyme",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "1142",
"journal-title": "Science",
"key": "10.1016/j.compbiomed.2024.107942_bib24",
"volume": "373",
"year": "2021"
},
{
"DOI": "10.1126/science.abg5827",
"article-title": "Masitinib is a broad coronavirus 3CL inhibitor that blocks replication of SARS-CoV-2",
"author": "Drayman",
"doi-asserted-by": "crossref",
"first-page": "931",
"journal-title": "Science",
"key": "10.1016/j.compbiomed.2024.107942_bib25",
"volume": "373",
"year": "2021"
},
{
"DOI": "10.1038/s41467-020-20542-0",
"article-title": "Mechanism of SARS-CoV-2 polymerase stalling by remdesivir",
"author": "Kokic",
"doi-asserted-by": "crossref",
"first-page": "279",
"journal-title": "Nat. Commun.",
"key": "10.1016/j.compbiomed.2024.107942_bib26",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.antiviral.2022.105389",
"article-title": "Punicalagin as an allosteric NSP13 helicase inhibitor potently suppresses SARS-CoV-2 replication in vitro",
"author": "Lu",
"doi-asserted-by": "crossref",
"journal-title": "Antivir. Res.",
"key": "10.1016/j.compbiomed.2024.107942_bib27",
"volume": "206",
"year": "2022"
},
{
"DOI": "10.1016/j.ijbiomac.2020.12.038",
"article-title": "Ritonavir may inhibit exoribonuclease activity of nsp14 from the SARS-CoV-2 virus and potentiate the activity of chain terminating drugs",
"author": "Narayanan",
"doi-asserted-by": "crossref",
"first-page": "272",
"journal-title": "Int. J. Biol. Macromol.",
"key": "10.1016/j.compbiomed.2024.107942_bib28",
"volume": "168",
"year": "2021"
},
{
"DOI": "10.1146/annurev-med-042220-012417",
"article-title": "COVID-19: inflammatory profile",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "65",
"journal-title": "Annu. Rev. Med.",
"key": "10.1016/j.compbiomed.2024.107942_bib29",
"volume": "73",
"year": "2022"
},
{
"DOI": "10.4049/jimmunol.1100917",
"article-title": "IL-17 boosts proinflammatory outcome of antiviral response in human cells",
"author": "Ryzhakov",
"doi-asserted-by": "crossref",
"first-page": "5357",
"journal-title": "J. Immunol.",
"key": "10.1016/j.compbiomed.2024.107942_bib30",
"volume": "187",
"year": "2011"
},
{
"DOI": "10.1111/resp.13875",
"article-title": "Targeting the interleukin-17 pathway to prevent acute respiratory distress syndrome associated with SARS-CoV-2 infection",
"author": "Wiche Salinas",
"doi-asserted-by": "crossref",
"first-page": "797",
"journal-title": "Respirology",
"key": "10.1016/j.compbiomed.2024.107942_bib31",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-1051-9",
"article-title": "An inflammatory cytokine signature predicts COVID-19 severity and survival",
"author": "Del Valle",
"doi-asserted-by": "crossref",
"first-page": "1636",
"journal-title": "Nat. Med.",
"key": "10.1016/j.compbiomed.2024.107942_bib32",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1016/S2665-9913(20)30309-X",
"article-title": "Accumulating evidence suggests anti-TNF therapy needs to be given trial priority in COVID-19 treatment",
"author": "Robinson",
"doi-asserted-by": "crossref",
"first-page": "e653",
"journal-title": "Lancet Rheumatol",
"key": "10.1016/j.compbiomed.2024.107942_bib33",
"volume": "2",
"year": "2020"
},
{
"article-title": "Oxidative stress and inflammatory markers in patients with COVID-19: potential role of RAGE, HMGB1, GFAP and COX-2 in disease severity",
"author": "Passos",
"journal-title": "Int. Immunopharm.",
"key": "10.1016/j.compbiomed.2024.107942_bib34",
"volume": "104",
"year": "2022"
},
{
"DOI": "10.1128/MMBR.00031-10",
"article-title": "Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases",
"author": "Cargnello",
"doi-asserted-by": "crossref",
"first-page": "50",
"journal-title": "Microbiol. Mol. Biol. Rev.",
"key": "10.1016/j.compbiomed.2024.107942_bib35",
"volume": "75",
"year": "2011"
}
],
"reference-count": 35,
"references-count": 35,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S001048252400026X"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Health Informatics",
"Computer Science Applications"
],
"subtitle": [],
"title": "All-trans retinoic acid acts as a dual-purpose inhibitor of SARS-CoV-2 infection and inflammation",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "169"
}