Retinoic Acid-Mediated Inhibition of Mouse Coronavirus Replication Is Dependent on IRF3 and CaMKK
et al., Viruses, doi:10.3390/v16010140, Jan 2024
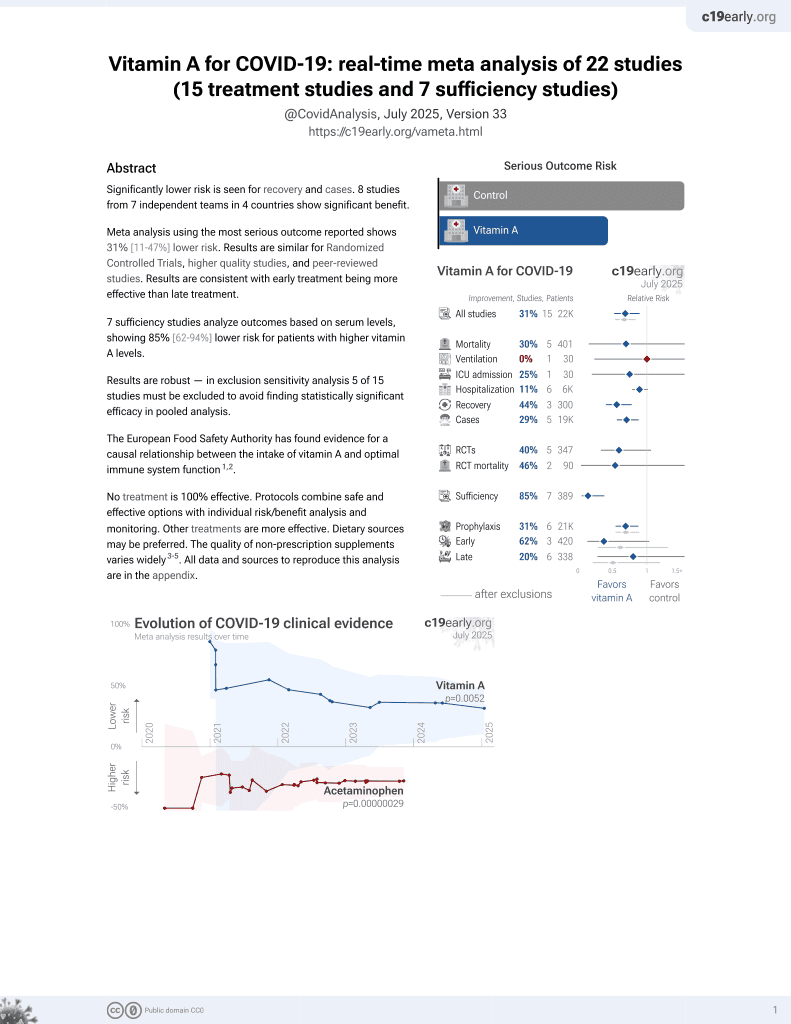
Vitamin A for COVID-19
49th treatment shown to reduce risk in
May 2023, now with p = 0.004 from 14 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
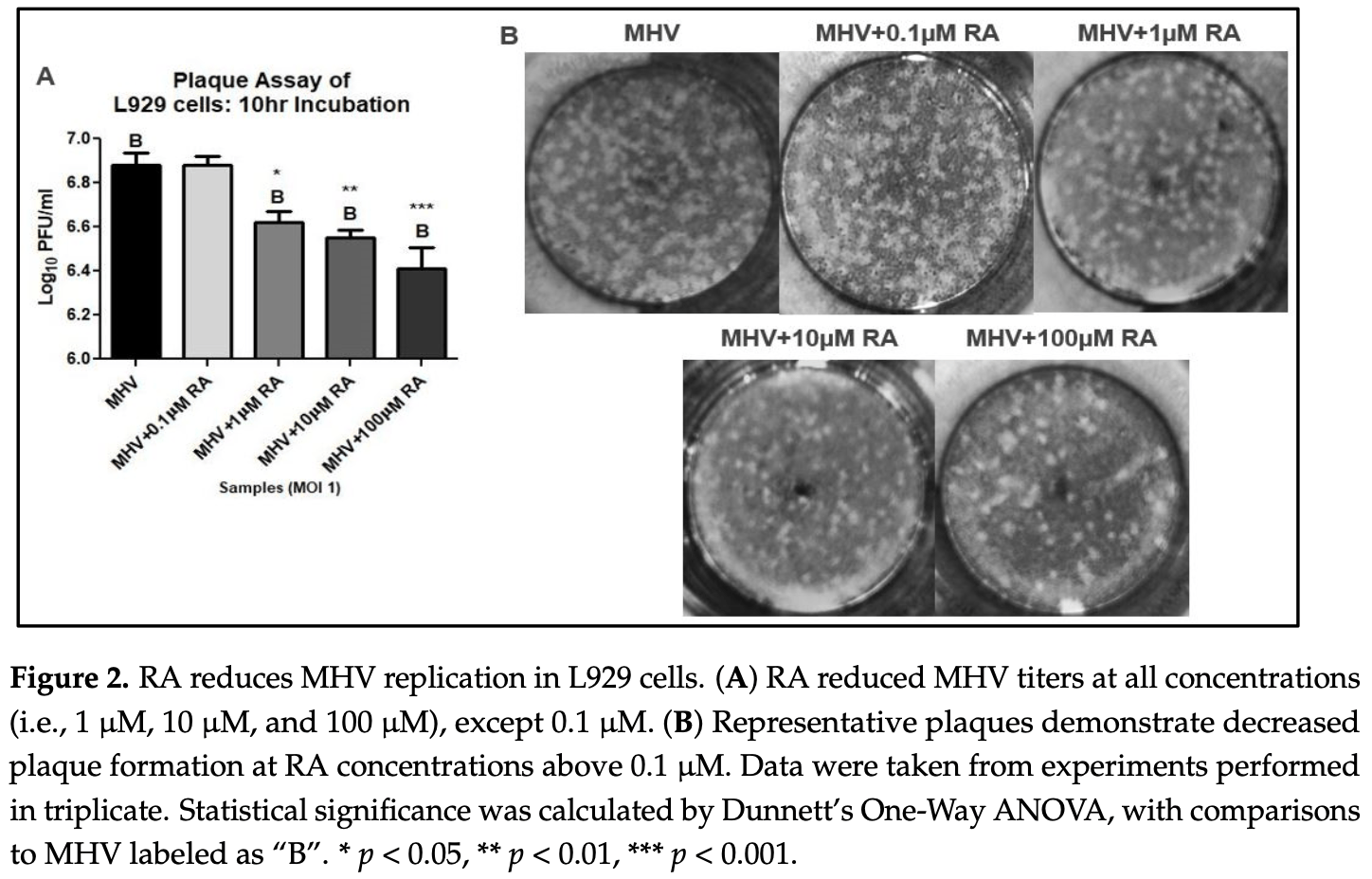
Mouse study showing inhibition of mouse coronavirus replication with all-trans retinoic acid (RA), a metabolite of vitamin A. Authors find that RA confers protection against infection by activating interferon responses in an IRF3-dependent manner, with reduced viral titers and nucleocapsid expression. RA induction of antiviral genes also relies on calcium/calmodulin kinase kinase (CaMKK) activity. The results demonstrate RA's potential as an antiviral therapeutic against coronaviruses through stimulation of innate immunity.
11 preclinical studies support the efficacy of vitamin A for COVID-19:
Vitamin A has been identified by the European Food Safety Authority (EFSA) as having sufficient evidence for a causal relationship between intake and optimal immune system function11-13.
Vitamin A has potent antiviral activity against SARS-CoV-2 in both human cell lines and human organoids of the lower respiratory tract (active metabolite all-trans retinoic acid, ATRA)8, is predicted to bind critical host and viral proteins for SARS-CoV-2 and may compensate for gene expression changes related to SARS-CoV-22-4, may be beneficial for COVID-19 via antiviral, anti-inflammatory, and immunomodulatory effects according to network pharmacology analysis5, reduces barrier compromise caused by TNF-α in Calu-3 cells7, inhibits mouse coronavirus replication10, may stimulate innate immunity by activating interferon responses in an IRF3-dependent manner (ATRA)10, may reduce excessive inflammation induced by SARS-CoV-22, shows SARS-CoV-2 antiviral activity In Vitro2,6,9 , is effective against multiple SARS-CoV-2 variants in Calu-3 cells9, and inhibits the entry and replication of SARS-CoV-2 via binding to ACE2 / 3CLpro / RdRp / helicase / 3'-to-5' exonuclease2.
1.
Voloudakis et al., A genetically based computational drug repurposing framework for rapid identification of candidate compounds: application to COVID-19, medRxiv, doi:10.1101/2025.01.10.25320348.
2.
Huang et al., All-trans retinoic acid acts as a dual-purpose inhibitor of SARS-CoV-2 infection and inflammation, Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2024.107942.
3.
Chakraborty et al., In-silico screening and in-vitro assay show the antiviral effect of Indomethacin against SARS-CoV-2, Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2022.105788.
4.
Pandya et al., Unravelling Vitamin B12 as a potential inhibitor against SARS-CoV-2: A computational approach, Informatics in Medicine Unlocked, doi:10.1016/j.imu.2022.100951.
5.
Li et al., Revealing the targets and mechanisms of vitamin A in the treatment of COVID-19, Aging, doi:10.18632/aging.103888.
6.
Moatasim et al., Potent Antiviral Activity of Vitamin B12 against Severe Acute Respiratory Syndrome Coronavirus 2, Middle East Respiratory Syndrome Coronavirus, and Human Coronavirus 229E, Microorganisms, doi:10.3390/microorganisms11112777.
7.
DiGuilio et al., The multiphasic TNF-α-induced compromise of Calu-3 airway epithelial barrier function, Experimental Lung Research, doi:10.1080/01902148.2023.2193637.
8.
Tong et al., A Retinol Derivative Inhibits SARS-CoV-2 Infection by Interrupting Spike-Mediated Cellular Entry, mBio, doi:10.1128/mbio.01485-22.
9.
Morita et al., All-Trans Retinoic Acid Exhibits Antiviral Effect against SARS-CoV-2 by Inhibiting 3CLpro Activity, Viruses, doi:10.3390/v13081669.
10.
Franco et al., Retinoic Acid-Mediated Inhibition of Mouse Coronavirus Replication Is Dependent on IRF3 and CaMKK, Viruses, doi:10.3390/v16010140.
11.
Galmés et al., Suboptimal Consumption of Relevant Immune System Micronutrients Is Associated with a Worse Impact of COVID-19 in Spanish Populations, Nutrients, doi:10.3390/nu14112254.
12.
Galmés (B) et al., Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework, Nutrients, doi:10.3390/nu12092738.
13.
EFSA, Scientific Opinion on the substantiation of health claims related to vitamin A and cell differentiation (ID 14), function of the immune system (ID 14), maintenance of skin and mucous membranes (ID 15, 17), maintenance of vision (ID 16), maintenance of bone (ID 13, 17), maintenance of teeth (ID 13, 17), maintenance of hair (ID 17), maintenance of nails (ID 17), metabolism of iron (ID 206), and protection of DNA, proteins and lipids from oxidative damage (ID 209) pursuant to Article 13(1) of Regulation (EC) No 1924/2006, EFSA Journal, doi:10.2903/j.efsa.2009.1221.
Franco et al., 18 Jan 2024, USA, peer-reviewed, 7 authors.
Contact: justin.franco@rockets.utoledo.edu (corresponding author), saurabh.chattopadhyay@uky.edu, kevin.pan@utoledo.edu.
Retinoic Acid-Mediated Inhibition of Mouse Coronavirus Replication Is Dependent on IRF3 and CaMKK
Viruses, doi:10.3390/v16010140
The ongoing COVID-19 pandemic has revealed the shortfalls in our understanding of how to treat coronavirus infections. With almost 7 million case fatalities of COVID-19 globally, the catalog of FDA-approved antiviral therapeutics is limited compared to other medications, such as antibiotics. All-trans retinoic acid (RA), or activated vitamin A, has been studied as a potential therapeutic against coronavirus infection because of its antiviral properties. Due to its impact on different signaling pathways, RA's mechanism of action during coronavirus infection has not been thoroughly described. To determine RA's mechanism of action, we examined its effect against a mouse coronavirus, mouse hepatitis virus strain A59 (MHV). We demonstrated that RA significantly decreased viral titers in infected mouse L929 fibroblasts and RAW 264.7 macrophages. The reduced viral titers were associated with a corresponding decrease in MHV nucleocapsid protein expression. Using interferon regulatory factor 3 (IRF3) knockout RAW 264.7 cells, we demonstrated that RA-induced suppression of MHV required IRF3 activity. RNA-seq analysis of wildtype and IRF3 knockout RAW cells showed that RA upregulated calcium/calmodulin (CaM) signaling proteins, such as CaM kinase kinase 1 (CaMKK1). When treated with a CaMKK inhibitor, RA was unable to upregulate IRF activation during MHV infection. In conclusion, our results demonstrate that RA-induced protection against coronavirus infection depends on IRF3 and CaMKK.
Supplementary Materials: The following supporting information can be downloaded at: https: //www.mdpi.com/article/10.3390/v16010140/s1, Figure S1 : 100 µM is non-toxic to mouse L929 cells, Figure S2
Conflicts of Interest: The authors declare no conflicts of interest.
References
Austenaa, Carlsen, Hollung, Blomhoff, Blomhoff, Retinoic acid dampens LPS-induced NF-kappaB activity: Results from human monoblasts and in vivo imaging of NF-kappaB reporter mice, J. Nutr. Biochem, doi:10.1016/j.jnutbio.2008.07.002
Axel, Frigge, Dittmann, Runge, Spyridopoulos et al., All-trans retinoic acid regulates proliferation, migration, differentiation, and extracellular matrix turnover of human arterial smooth muscle cells, Cardiovasc. Res, doi:10.1016/s0008-6363(00)00312-6
Bray, Pimentel, Melsted, Pachter, Near-optimal probabilistic RNA-seq quantification, Nat. Biotechnol, doi:10.1038/nbt.3519
Bremner, Shearer, Mccaffery, Retinoic acid and affective disorders: The evidence for an association, J. Clin. Psychiatry, doi:10.4088/JCP.10r05993
Brzozowski, Skelding, The Multi-Functional Calcium/Calmodulin Stimulated Protein Kinase (CaMK) Family: Emerging Targets for Anti-Cancer Therapeutic Intervention, Pharmaceuticals, doi:10.3390/ph12010008
Butchi, Hinton, Stohlman, Kapil, Fensterl et al., Ifit2 deficiency results in uncontrolled neurotropic coronavirus replication and enhanced encephalitis via impaired alpha/beta interferon induction in macrophages, J. Virol, doi:10.1128/JVI.02272-13
Chang, Liu, Chang, Chang, Middle East Respiratory Syndrome Coronavirus Nucleocapsid Protein Suppresses Type I and Type III Interferon Induction by Targeting RIG-I Signaling, J. Virol, doi:10.1128/JVI.00099-20
Chattopadhyay, Kuzmanovic, Zhang, Wetzel, Sen, Ubiquitination of the Transcription Factor IRF-3 Activates RIPA, the Apoptotic Pathway that Protects Mice from Viral Pathogenesis, Immunity, doi:10.1016/j.immuni.2016.04.009
Chen, Liu, Cao, Regulation of type I interferon signaling in immunity and inflammation: A comprehensive review, J. Autoimmun, doi:10.1016/j.jaut.2017.03.008
Chen, Xiao, Hu, Ge, Tian et al., SARS-CoV-2 Nucleocapsid Protein Interacts with RIG-I and Represses RIG-Mediated IFN-beta Production, Viruses, doi:10.3390/v13010047
Das Sarma, Burrows, Rayman, Hwang, Kundu et al., Ifit2 deficiency restricts microglial activation and leukocyte migration following murine coronavirus (m-CoV) CNS infection, PLoS Pathog, doi:10.1371/journal.ppat.1009034
Diamond, Farzan, The broad-spectrum antiviral functions of IFIT and IFITM proteins, Nat. Rev. Immunol, doi:10.1038/nri3344
Ding, Fang, Yuan, Zhao, Wang et al., The nucleocapsid proteins of mouse hepatitis virus and severe acute respiratory syndrome coronavirus share the same IFN-beta antagonizing mechanism: Attenuation of PACT-mediated RIG-I/MDA5 activation, Oncotarget, doi:10.18632/oncotarget.17912
Featherstone, Brown, Chitlapilly Dass, Murine Hepatitis Virus, a Biosafety Level 2 Model for SARS-CoV-2, Can Remain Viable on Meat and Meat Packaging Materials for at Least 48 Hours, Microbiol. Spectr, doi:10.1128/spectrum.01862-22
Franco, Chattopadhyay, Pan, How Different Pathologies Are Affected by IFIT Expression, Viruses, doi:10.3390/v15020342
Glanz, Chakravarty, Varghese, Kottapalli, Fan et al., Transcriptional and Non-Transcriptional Activation, Posttranslational Modifications, and Antiviral Functions of Interferon Regulatory Factor 3 and Viral Antagonism by the SARS-Coronavirus, Viruses, doi:10.3390/v13040575
Grana, Ghosn, Evrenoglou, Jarde, Minozzi et al., Efficacy and safety of COVID-19 vaccines, Cochrane Database Syst. Rev, doi:10.1002/14651858.CD015477
Gu, Eils, Schlesner, Complex heatmaps reveal patterns and correlations in multidimensional genomic data, Bioinformatics, doi:10.1093/bioinformatics/btw313
Guest, Deszo, Hartman, York, Kelley et al., Ca 2+ /calmodulin-dependent kinase kinase alpha is expressed by monocytic cells and regulates the activation profile, PLoS ONE, doi:10.1371/journal.pone.0001606
Gulick, Masure, Pau, Aberg, Adimora et al., Coronavirus Disease 2019 (COVID-19) Treatment Guidelines, Natl. Inst. Health
Hamamoto, Fukuda, Ishimura, Rumi, Kazumori et al., 9-cis retinoic acid enhances the antiviral effect of interferon on hepatitis C virus replication through increased expression of type I interferon receptor, J. Lab. Clin. Med, doi:10.1067/mlc.2003.8
Hawley, Selbert, Goldstein, Edelman, Carling et al., 5 ′ -AMP activates the AMP-activated protein kinase cascade, and Ca 2+ /calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms, J. Biol. Chem, doi:10.1074/jbc.270.45.27186
Hook, Means, Ca(2+)/CaM-dependent kinases: From activation to function, Annu. Rev. Pharmacol. Toxicol, doi:10.1146/annurev.pharmtox.41.1.471
Hu, Van Dam, Wang, Lucassen, Zhou, Retinoic acid and depressive disorders: Evidence and possible neurobiological mechanisms, Neurosci. Biobehav. Rev, doi:10.1016/j.neubiorev.2020.02.013
Hurley, Anderson, Franzone, Kemp, Means et al., The Ca 2+ /calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases, J. Biol. Chem, doi:10.1074/jbc.M503824200
Ivashkiv, Donlin, Regulation of type I interferon responses, Nat. Rev. Immunol, doi:10.1038/nri3581
Kaitsuka, Li, Nakamura, Takao, Miyakawa et al., Forebrain-specific constitutively active CaMKKalpha transgenic mice show deficits in hippocampus-dependent long-term memory, Neurobiol. Learn. Mem
Kasuga, Zhu, Jang, Yoo, Innate immune sensing of coronavirus and viral evasion strategies, Exp. Mol. Med, doi:10.1038/s12276-021-00602-1
Korner, Majjouti, Alcazar, Mahabir, Of Mice and Men: The Coronavirus MHV and Mouse Models as a Translational Approach to Understand SARS-CoV-2, Viruses, doi:10.3390/v12080880
Lei, Dong, Ma, Wang, Xiao et al., Activation and evasion of type I interferon responses by SARS-CoV-2, Nat. Commun, doi:10.1038/s41467-020-17665-9
Liu, Cai, Wu, Cong, Chen et al., Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation, Science, doi:10.1126/science.aaa2630
Maeda, Yamaguchi, Hijikata, Morita, Tanaka et al., All-trans retinoic acid attacks reverse transcriptase resulting in inhibition of HIV-1 replication, Hematology, doi:10.1080/10245330701255130
Marcelo, Means, York, The Ca(2+)/Calmodulin/CaMKK2 Axis: Nature's Metabolic CaMshaft, Trends Endocrinol. Metab, doi:10.1016/j.tem.2016.06.001
Merad, Blish, Sallusto, Iwasaki, The immunology and immunopathology of COVID-19, Science, doi:10.1126/science.abm8108
Merhi, Alvarez-Valadez, Trepiana, Lescoat, Groppi et al., Targeting CAMKK2 and SOC Channels as a Novel Therapeutic Approach for Sensitizing Acute Promyelocytic Leukemia Cells to All-Trans Retinoic Acid, Cells, doi:10.3390/cells10123364
Morita, Miyakawa, Jeremiah, Yamaoka, Sada et al., All-Trans Retinoic Acid Exhibits Antiviral Effect against SARS-CoV-2 by Inhibiting 3CLpro Activity, Viruses, doi:10.3390/v13081669
Njar, Gediya, Purushottamachar, Chopra, Vasaitis et al., Retinoic acid metabolism blocking agents (RAMBAs) for treatment of cancer and dermatological diseases, Bioorg. Med. Chem, doi:10.1016/j.bmc.2006.02.041
Paidas, Mohamed, Norenberg, Saad, Barry et al., Multi-Organ Histopathological Changes in a Mouse Hepatitis Virus Model of COVID-19, Viruses, doi:10.3390/v13091703
Pereira Oliveira, Kroon, Mouse hepatitis virus: A betacoronavirus model to study the virucidal activity of air disinfection equipment on surface contamination, J. Virol. Methods, doi:10.1016/j.jviromet.2021.114274
Pino-Lagos, Guo, Noelle, Retinoic acid: A key player in immunity, Biofactors, doi:10.1002/biof.117
Plikus, Wang, Sinha, Forte, Thompson et al., Fibroblasts: Origins, definitions, and functions in health and disease, Cell, doi:10.1016/j.cell.2021.06.024
Popli, Chakravarty, Fan, Glanz, Aras et al., IRF3 inhibits nuclear translocation of NF-kappaB to prevent viral inflammation, Proc. Natl. Acad. Sci, doi:10.1073/pnas.2121385119
Prantner, Perkins, Vogel, AMP-activated Kinase (AMPK) Promotes Innate Immunity and Antiviral Defense through Modulation of Stimulator of Interferon Genes (STING) Signaling, J. Biol. Chem, doi:10.1074/jbc.M116.763268
Robinson, Mccarthy, Smyth, Edger, A Bioconductor package for differential expression analysis of digital gene expression data, Bioinformatics, doi:10.1093/bioinformatics/btp616
Salaciak, Koszalka, Zmudzka, Pytka, The Calcium/Calmodulin-Dependent Kinases II and IV as Therapeutic Targets in Neurodegenerative and Neuropsychiatric Disorders, Int. J. Mol. Sci, doi:10.3390/ijms22094307
Schultze, Aschenbrenner, COVID-19 and the human innate immune system, Cell, doi:10.1016/j.cell.2021.02.029
Soneson, Love, Robinson, Differential analyses for RNA-seq: Transcript-level estimates improve gene-level inferences, F1000Res, doi:10.12688/f1000research.7563.1
Soye, Trottier, Di Lenardo, Restori, Reichman et al., In vitro inhibition of mumps virus by retinoids, Virol. J, doi:10.1186/1743-422X-10-337
Soye, Trottier, Richardson, Ward, Miller et al., RIG-I is required for the inhibition of measles virus by retinoids, PLoS ONE, doi:10.1371/journal.pone.0022323
Tokumitsu, Sakagami, Molecular Mechanisms Underlying Ca(2+)/Calmodulin-Dependent Protein Kinase Kinase Signal Transduction, Int. J. Mol. Sci, doi:10.3390/ijms231911025
Wang, Zhang, Du, Du, Zhao et al., Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial, Lancet, doi:10.1016/S0140-6736(20)31022-9
Wang, Zhang, Wu, Rong, Guo, M2b macrophage polarization and its roles in diseases, J. Leukoc. Biol, doi:10.1002/JLB.3RU1018-378RR
Wiersinga, Rhodes, Cheng, Peacock, Prescott et al., Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review, JAMA, doi:10.1001/jama.2020.12839
Wolf, Wolf, Bello, Maccari, Nasi, Molecular evolution of SARS-CoV-2 from December 2019 to August 2022, J. Med. Virol, doi:10.1002/jmv.28366
Yang, Du, Chen, Zhao, Yang et al., Coronavirus MHV-A59 infects the lung and causes severe pneumonia in C57BL/6 mice, Virol. Sin, doi:10.1007/s12250-014-3530-y
Ye, Hauns, Langland, Jacobs, Hogue, Mouse hepatitis coronavirus A59 nucleocapsid protein is a type I interferon antagonist, J. Virol, doi:10.1128/JVI.01634-06
Yuan, Chu, Chan, Ye, Wen et al., SREBP-dependent lipidomic reprogramming as a broad-spectrum antiviral target, Nat. Commun, doi:10.1038/s41467-018-08015-x
Zhu, Zhang, Wang, Li, Yang et al., A Novel Coronavirus from Patients with Pneumonia in China, N. Engl. J. Med, doi:10.1056/NEJMoa2001017
DOI record:
{
"DOI": "10.3390/v16010140",
"ISSN": [
"1999-4915"
],
"URL": "http://dx.doi.org/10.3390/v16010140",
"abstract": "<jats:p>The ongoing COVID-19 pandemic has revealed the shortfalls in our understanding of how to treat coronavirus infections. With almost 7 million case fatalities of COVID-19 globally, the catalog of FDA-approved antiviral therapeutics is limited compared to other medications, such as antibiotics. All-trans retinoic acid (RA), or activated vitamin A, has been studied as a potential therapeutic against coronavirus infection because of its antiviral properties. Due to its impact on different signaling pathways, RA’s mechanism of action during coronavirus infection has not been thoroughly described. To determine RA’s mechanism of action, we examined its effect against a mouse coronavirus, mouse hepatitis virus strain A59 (MHV). We demonstrated that RA significantly decreased viral titers in infected mouse L929 fibroblasts and RAW 264.7 macrophages. The reduced viral titers were associated with a corresponding decrease in MHV nucleocapsid protein expression. Using interferon regulatory factor 3 (IRF3) knockout RAW 264.7 cells, we demonstrated that RA-induced suppression of MHV required IRF3 activity. RNA-seq analysis of wildtype and IRF3 knockout RAW cells showed that RA upregulated calcium/calmodulin (CaM) signaling proteins, such as CaM kinase kinase 1 (CaMKK1). When treated with a CaMKK inhibitor, RA was unable to upregulate IRF activation during MHV infection. In conclusion, our results demonstrate that RA-induced protection against coronavirus infection depends on IRF3 and CaMKK.</jats:p>",
"alternative-id": [
"v16010140"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-5503-8928",
"affiliation": [
{
"name": "Department of Medical Microbiology and Immunology, University of Toledo College of Medicine and Life Sciences, Toledo, OH 43614, USA"
}
],
"authenticated-orcid": false,
"family": "Franco",
"given": "Justin H.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Medical Microbiology and Immunology, University of Toledo College of Medicine and Life Sciences, Toledo, OH 43614, USA"
}
],
"family": "Harris",
"given": "Ryan A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4868-4002",
"affiliation": [
{
"name": "Department of Neurosciences and Neurological Disorders, University of Toledo College of Medicine and Life Sciences, Toledo, OH 43614, USA"
}
],
"authenticated-orcid": false,
"family": "Ryan",
"given": "William G.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3819-8548",
"affiliation": [
{
"name": "Department of Medical Microbiology and Immunology, University of Toledo College of Medicine and Life Sciences, Toledo, OH 43614, USA"
}
],
"authenticated-orcid": false,
"family": "Taylor",
"given": "Roger Travis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Neurosciences and Neurological Disorders, University of Toledo College of Medicine and Life Sciences, Toledo, OH 43614, USA"
}
],
"family": "McCullumsmith",
"given": "Robert E.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2930-1523",
"affiliation": [
{
"name": "Department of Medical Microbiology and Immunology, University of Toledo College of Medicine and Life Sciences, Toledo, OH 43614, USA"
},
{
"name": "Department of Microbiology Immunology and Molecular Genetics, University of Kentucky College of Medicine, Lexington, KY 40536, USA"
}
],
"authenticated-orcid": false,
"family": "Chattopadhyay",
"given": "Saurabh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medical Microbiology and Immunology, University of Toledo College of Medicine and Life Sciences, Toledo, OH 43614, USA"
}
],
"family": "Pan",
"given": "Zhixing K.",
"sequence": "additional"
}
],
"container-title": "Viruses",
"container-title-short": "Viruses",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
1,
18
]
],
"date-time": "2024-01-18T11:41:22Z",
"timestamp": 1705578082000
},
"deposited": {
"date-parts": [
[
2024,
1,
18
]
],
"date-time": "2024-01-18T11:45:50Z",
"timestamp": 1705578350000
},
"funder": [
{
"award": [
"NIGMS T32-G-RISE 1T32GM144873-01",
"AI155545",
"AI165521"
],
"name": "US National Institutes of Health"
}
],
"indexed": {
"date-parts": [
[
2024,
1,
19
]
],
"date-time": "2024-01-19T00:20:07Z",
"timestamp": 1705623607495
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
1,
18
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
18
]
],
"date-time": "2024-01-18T00:00:00Z",
"timestamp": 1705536000000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1999-4915/16/1/140/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "140",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2024,
1,
18
]
]
},
"published-online": {
"date-parts": [
[
2024,
1,
18
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1002/jmv.28366",
"article-title": "Molecular evolution of SARS-CoV-2 from December 2019 to August 2022",
"author": "Wolf",
"doi-asserted-by": "crossref",
"first-page": "e28366",
"journal-title": "J. Med. Virol.",
"key": "ref_1",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2001017",
"article-title": "A Novel Coronavirus from Patients with Pneumonia in China, 2019",
"author": "Zhu",
"doi-asserted-by": "crossref",
"first-page": "727",
"journal-title": "N. Engl. J. Med.",
"key": "ref_2",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.12839",
"article-title": "Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review",
"author": "Wiersinga",
"doi-asserted-by": "crossref",
"first-page": "782",
"journal-title": "JAMA",
"key": "ref_3",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1126/science.abm8108",
"article-title": "The immunology and immunopathology of COVID-19",
"author": "Merad",
"doi-asserted-by": "crossref",
"first-page": "1122",
"journal-title": "Science",
"key": "ref_4",
"volume": "375",
"year": "2022"
},
{
"DOI": "10.3390/v15020342",
"doi-asserted-by": "crossref",
"key": "ref_5",
"unstructured": "Franco, J.H., Chattopadhyay, S., and Pan, Z.K. (2023). How Different Pathologies Are Affected by IFIT Expression. Viruses, 15."
},
{
"DOI": "10.1016/j.jaut.2017.03.008",
"article-title": "Regulation of type I interferon signaling in immunity and inflammation: A comprehensive review",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "J. Autoimmun.",
"key": "ref_6",
"volume": "83",
"year": "2017"
},
{
"DOI": "10.1038/nri3581",
"article-title": "Regulation of type I interferon responses",
"author": "Ivashkiv",
"doi-asserted-by": "crossref",
"first-page": "36",
"journal-title": "Nat. Rev. Immunol.",
"key": "ref_7",
"volume": "14",
"year": "2014"
},
{
"DOI": "10.3390/v13040575",
"doi-asserted-by": "crossref",
"key": "ref_8",
"unstructured": "Glanz, A., Chakravarty, S., Varghese, M., Kottapalli, A., Fan, S., Chakravarti, R., and Chattopadhyay, S. (2021). Transcriptional and Non-Transcriptional Activation, Posttranslational Modifications, and Antiviral Functions of Interferon Regulatory Factor 3 and Viral Antagonism by the SARS-Coronavirus. Viruses, 13."
},
{
"DOI": "10.1016/j.immuni.2016.04.009",
"article-title": "Ubiquitination of the Transcription Factor IRF-3 Activates RIPA, the Apoptotic Pathway that Protects Mice from Viral Pathogenesis",
"author": "Chattopadhyay",
"doi-asserted-by": "crossref",
"first-page": "1151",
"journal-title": "Immunity",
"key": "ref_9",
"volume": "44",
"year": "2016"
},
{
"DOI": "10.1073/pnas.2121385119",
"article-title": "IRF3 inhibits nuclear translocation of NF-kappaB to prevent viral inflammation",
"author": "Popli",
"doi-asserted-by": "crossref",
"first-page": "e2121385119",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_10",
"volume": "119",
"year": "2022"
},
{
"DOI": "10.1038/s12276-021-00602-1",
"article-title": "Innate immune sensing of coronavirus and viral evasion strategies",
"author": "Kasuga",
"doi-asserted-by": "crossref",
"first-page": "723",
"journal-title": "Exp. Mol. Med.",
"key": "ref_11",
"volume": "53",
"year": "2021"
},
{
"DOI": "10.1038/s41467-020-17665-9",
"article-title": "Activation and evasion of type I interferon responses by SARS-CoV-2",
"author": "Lei",
"doi-asserted-by": "crossref",
"first-page": "3810",
"journal-title": "Nat. Commun.",
"key": "ref_12",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3390/v13010047",
"doi-asserted-by": "crossref",
"key": "ref_13",
"unstructured": "Chen, K., Xiao, F., Hu, D., Ge, W., Tian, M., Wang, W., Pan, P., Wu, K., and Wu, J. (2020). SARS-CoV-2 Nucleocapsid Protein Interacts with RIG-I and Represses RIG-Mediated IFN-beta Production. Viruses, 13."
},
{
"DOI": "10.1128/JVI.00099-20",
"article-title": "Middle East Respiratory Syndrome Coronavirus Nucleocapsid Protein Suppresses Type I and Type III Interferon Induction by Targeting RIG-I Signaling",
"author": "Chang",
"doi-asserted-by": "crossref",
"first-page": "e00099-20",
"journal-title": "J. Virol.",
"key": "ref_14",
"volume": "94",
"year": "2020"
},
{
"DOI": "10.18632/oncotarget.17912",
"article-title": "The nucleocapsid proteins of mouse hepatitis virus and severe acute respiratory syndrome coronavirus share the same IFN-beta antagonizing mechanism: Attenuation of PACT-mediated RIG-I/MDA5 activation",
"author": "Ding",
"doi-asserted-by": "crossref",
"first-page": "49655",
"journal-title": "Oncotarget",
"key": "ref_15",
"volume": "8",
"year": "2017"
},
{
"DOI": "10.1128/JVI.01634-06",
"article-title": "Mouse hepatitis coronavirus A59 nucleocapsid protein is a type I interferon antagonist",
"author": "Ye",
"doi-asserted-by": "crossref",
"first-page": "2554",
"journal-title": "J. Virol.",
"key": "ref_16",
"volume": "81",
"year": "2007"
},
{
"article-title": "Efficacy and safety of COVID-19 vaccines",
"author": "Grana",
"first-page": "CD015477",
"journal-title": "Cochrane Database Syst. Rev.",
"key": "ref_17",
"volume": "12",
"year": "2022"
},
{
"article-title": "Coronavirus Disease 2019 (COVID-19) Treatment Guidelines",
"author": "Gulick",
"first-page": "469",
"journal-title": "Natl. Inst. Health",
"key": "ref_18",
"volume": "1",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"article-title": "Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1569",
"journal-title": "Lancet",
"key": "ref_19",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.bmc.2006.02.041",
"article-title": "Retinoic acid metabolism blocking agents (RAMBAs) for treatment of cancer and dermatological diseases",
"author": "Njar",
"doi-asserted-by": "crossref",
"first-page": "4323",
"journal-title": "Bioorg. Med. Chem.",
"key": "ref_20",
"volume": "14",
"year": "2006"
},
{
"DOI": "10.1002/biof.117",
"article-title": "Retinoic acid: A key player in immunity",
"author": "Guo",
"doi-asserted-by": "crossref",
"first-page": "430",
"journal-title": "Biofactors",
"key": "ref_21",
"volume": "36",
"year": "2010"
},
{
"DOI": "10.1371/journal.pone.0022323",
"doi-asserted-by": "crossref",
"key": "ref_22",
"unstructured": "Soye, K.J., Trottier, C., Richardson, C.D., Ward, B.J., and Miller, W.H. (2011). RIG-I is required for the inhibition of measles virus by retinoids. PLoS ONE, 6."
},
{
"DOI": "10.1186/1743-422X-10-337",
"article-title": "In vitro inhibition of mumps virus by retinoids",
"author": "Soye",
"doi-asserted-by": "crossref",
"first-page": "337",
"journal-title": "Virol. J.",
"key": "ref_23",
"volume": "10",
"year": "2013"
},
{
"DOI": "10.1067/mlc.2003.8",
"article-title": "9-cis retinoic acid enhances the antiviral effect of interferon on hepatitis C virus replication through increased expression of type I interferon receptor",
"author": "Hamamoto",
"doi-asserted-by": "crossref",
"first-page": "58",
"journal-title": "J. Lab. Clin. Med.",
"key": "ref_24",
"volume": "141",
"year": "2003"
},
{
"DOI": "10.1080/10245330701255130",
"article-title": "All-trans retinoic acid attacks reverse transcriptase resulting in inhibition of HIV-1 replication",
"author": "Maeda",
"doi-asserted-by": "crossref",
"first-page": "263",
"journal-title": "Hematology",
"key": "ref_25",
"volume": "12",
"year": "2007"
},
{
"DOI": "10.3390/v13081669",
"doi-asserted-by": "crossref",
"key": "ref_26",
"unstructured": "Morita, T., Miyakawa, K., Jeremiah, S.S., Yamaoka, Y., Sada, M., Kuniyoshi, T., Yang, J., Kimura, H., and Ryo, A. (2021). All-Trans Retinoic Acid Exhibits Antiviral Effect against SARS-CoV-2 by Inhibiting 3CLpro Activity. Viruses, 13."
},
{
"DOI": "10.1038/s41467-018-08015-x",
"article-title": "SREBP-dependent lipidomic reprogramming as a broad-spectrum antiviral target",
"author": "Yuan",
"doi-asserted-by": "crossref",
"first-page": "120",
"journal-title": "Nat. Commun.",
"key": "ref_27",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1038/nbt.3519",
"article-title": "Near-optimal probabilistic RNA-seq quantification",
"author": "Bray",
"doi-asserted-by": "crossref",
"first-page": "525",
"journal-title": "Nat. Biotechnol.",
"key": "ref_28",
"volume": "34",
"year": "2016"
},
{
"DOI": "10.12688/f1000research.7563.1",
"article-title": "Differential analyses for RNA-seq: Transcript-level estimates improve gene-level inferences",
"author": "Soneson",
"doi-asserted-by": "crossref",
"first-page": "1521",
"journal-title": "F1000Res",
"key": "ref_29",
"volume": "4",
"year": "2015"
},
{
"DOI": "10.1093/bioinformatics/btp616",
"article-title": "edgeR: A Bioconductor package for differential expression analysis of digital gene expression data",
"author": "Robinson",
"doi-asserted-by": "crossref",
"first-page": "139",
"journal-title": "Bioinformatics",
"key": "ref_30",
"volume": "26",
"year": "2010"
},
{
"DOI": "10.1093/bioinformatics/btw313",
"article-title": "Complex heatmaps reveal patterns and correlations in multidimensional genomic data",
"author": "Gu",
"doi-asserted-by": "crossref",
"first-page": "2847",
"journal-title": "Bioinformatics",
"key": "ref_31",
"volume": "32",
"year": "2016"
},
{
"DOI": "10.3390/v12080880",
"doi-asserted-by": "crossref",
"key": "ref_32",
"unstructured": "Korner, R.W., Majjouti, M., Alcazar, M.A.A., and Mahabir, E. (2020). Of Mice and Men: The Coronavirus MHV and Mouse Models as a Translational Approach to Understand SARS-CoV-2. Viruses, 12."
},
{
"DOI": "10.1016/j.jviromet.2021.114274",
"article-title": "Mouse hepatitis virus: A betacoronavirus model to study the virucidal activity of air disinfection equipment on surface contamination",
"author": "Kroon",
"doi-asserted-by": "crossref",
"first-page": "114274",
"journal-title": "J. Virol. Methods",
"key": "ref_33",
"volume": "297",
"year": "2021"
},
{
"DOI": "10.1128/spectrum.01862-22",
"doi-asserted-by": "crossref",
"key": "ref_34",
"unstructured": "Featherstone, A.B., Brown, A.C., and Chitlapilly Dass, S. (2022). Murine Hepatitis Virus, a Biosafety Level 2 Model for SARS-CoV-2, Can Remain Viable on Meat and Meat Packaging Materials for at Least 48 Hours. Microbiol. Spectr., 10."
},
{
"DOI": "10.3390/v13091703",
"doi-asserted-by": "crossref",
"key": "ref_35",
"unstructured": "Paidas, M.J., Mohamed, A.B., Norenberg, M.D., Saad, A., Barry, A.F., Colon, C., Kenyon, N.S., and Jayakumar, A.R. (2021). Multi-Organ Histopathological Changes in a Mouse Hepatitis Virus Model of COVID-19. Viruses, 13."
},
{
"DOI": "10.1007/s12250-014-3530-y",
"article-title": "Coronavirus MHV-A59 infects the lung and causes severe pneumonia in C57BL/6 mice",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "393",
"journal-title": "Virol. Sin.",
"key": "ref_36",
"volume": "29",
"year": "2014"
},
{
"DOI": "10.1016/j.cell.2021.06.024",
"article-title": "Fibroblasts: Origins, definitions, and functions in health and disease",
"author": "Plikus",
"doi-asserted-by": "crossref",
"first-page": "3852",
"journal-title": "Cell",
"key": "ref_37",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2021.02.029",
"article-title": "COVID-19 and the human innate immune system",
"author": "Schultze",
"doi-asserted-by": "crossref",
"first-page": "1671",
"journal-title": "Cell",
"key": "ref_38",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1016/S0008-6363(00)00312-6",
"article-title": "All-trans retinoic acid regulates proliferation, migration, differentiation, and extracellular matrix turnover of human arterial smooth muscle cells",
"author": "Axel",
"doi-asserted-by": "crossref",
"first-page": "851",
"journal-title": "Cardiovasc. Res.",
"key": "ref_39",
"volume": "49",
"year": "2001"
},
{
"DOI": "10.1016/j.tem.2016.06.001",
"article-title": "The Ca(2+)/Calmodulin/CaMKK2 Axis: Nature’s Metabolic CaMshaft",
"author": "Marcelo",
"doi-asserted-by": "crossref",
"first-page": "706",
"journal-title": "Trends Endocrinol. Metab.",
"key": "ref_40",
"volume": "27",
"year": "2016"
},
{
"DOI": "10.1146/annurev.pharmtox.41.1.471",
"article-title": "Ca(2+)/CaM-dependent kinases: From activation to function",
"author": "Hook",
"doi-asserted-by": "crossref",
"first-page": "471",
"journal-title": "Annu. Rev. Pharmacol. Toxicol.",
"key": "ref_41",
"volume": "41",
"year": "2001"
},
{
"DOI": "10.3390/ijms231911025",
"doi-asserted-by": "crossref",
"key": "ref_42",
"unstructured": "Tokumitsu, H., and Sakagami, H. (2022). Molecular Mechanisms Underlying Ca(2+)/Calmodulin-Dependent Protein Kinase Kinase Signal Transduction. Int. J. Mol. Sci., 23."
},
{
"DOI": "10.3390/ph12010008",
"doi-asserted-by": "crossref",
"key": "ref_43",
"unstructured": "Brzozowski, J.S., and Skelding, K.A. (2019). The Multi-Functional Calcium/Calmodulin Stimulated Protein Kinase (CaMK) Family: Emerging Targets for Anti-Cancer Therapeutic Intervention. Pharmaceuticals, 12."
},
{
"DOI": "10.1371/journal.pone.0001606",
"doi-asserted-by": "crossref",
"key": "ref_44",
"unstructured": "Guest, C.B., Deszo, E.L., Hartman, M.E., York, J.M., Kelley, K.W., and Freund, G.G. (2008). Ca2+/calmodulin-dependent kinase kinase alpha is expressed by monocytic cells and regulates the activation profile. PLoS ONE, 3."
},
{
"DOI": "10.1016/j.jnutbio.2008.07.002",
"article-title": "Retinoic acid dampens LPS-induced NF-kappaB activity: Results from human monoblasts and in vivo imaging of NF-kappaB reporter mice",
"author": "Austenaa",
"doi-asserted-by": "crossref",
"first-page": "726",
"journal-title": "J. Nutr. Biochem.",
"key": "ref_45",
"volume": "20",
"year": "2009"
},
{
"DOI": "10.1038/nri3344",
"article-title": "The broad-spectrum antiviral functions of IFIT and IFITM proteins",
"author": "Diamond",
"doi-asserted-by": "crossref",
"first-page": "46",
"journal-title": "Nat. Rev. Immunol.",
"key": "ref_46",
"volume": "13",
"year": "2013"
},
{
"DOI": "10.1128/JVI.02272-13",
"article-title": "Ifit2 deficiency results in uncontrolled neurotropic coronavirus replication and enhanced encephalitis via impaired alpha/beta interferon induction in macrophages",
"author": "Butchi",
"doi-asserted-by": "crossref",
"first-page": "1051",
"journal-title": "J. Virol.",
"key": "ref_47",
"volume": "88",
"year": "2014"
},
{
"DOI": "10.1371/journal.ppat.1009034",
"doi-asserted-by": "crossref",
"key": "ref_48",
"unstructured": "Das Sarma, J., Burrows, A., Rayman, P., Hwang, M.H., Kundu, S., Sharma, N., Bergmann, C., and Sen, G.C. (2020). Ifit2 deficiency restricts microglial activation and leukocyte migration following murine coronavirus (m-CoV) CNS infection. PLoS Pathog., 16."
},
{
"DOI": "10.1074/jbc.270.45.27186",
"article-title": "5′-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms",
"author": "Hawley",
"doi-asserted-by": "crossref",
"first-page": "27186",
"journal-title": "J. Biol. Chem.",
"key": "ref_49",
"volume": "270",
"year": "1995"
},
{
"DOI": "10.1074/jbc.M503824200",
"article-title": "The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases",
"author": "Hurley",
"doi-asserted-by": "crossref",
"first-page": "29060",
"journal-title": "J. Biol. Chem.",
"key": "ref_50",
"volume": "280",
"year": "2005"
},
{
"DOI": "10.1074/jbc.M116.763268",
"article-title": "AMP-activated Kinase (AMPK) Promotes Innate Immunity and Antiviral Defense through Modulation of Stimulator of Interferon Genes (STING) Signaling",
"author": "Prantner",
"doi-asserted-by": "crossref",
"first-page": "292",
"journal-title": "J. Biol. Chem.",
"key": "ref_51",
"volume": "292",
"year": "2017"
},
{
"DOI": "10.1126/science.aaa2630",
"article-title": "Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "aaa2630",
"journal-title": "Science",
"key": "ref_52",
"volume": "347",
"year": "2015"
},
{
"DOI": "10.1002/JLB.3RU1018-378RR",
"article-title": "M2b macrophage polarization and its roles in diseases",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "345",
"journal-title": "J. Leukoc. Biol.",
"key": "ref_53",
"volume": "106",
"year": "2019"
},
{
"DOI": "10.3390/cells10123364",
"doi-asserted-by": "crossref",
"key": "ref_54",
"unstructured": "Merhi, F., Alvarez-Valadez, K., Trepiana, J., Lescoat, C., Groppi, A., Dupuy, J.W., Soubeyran, P., Kroemer, G., Vacher, P., and Djavaheri-Mergny, M. (2021). Targeting CAMKK2 and SOC Channels as a Novel Therapeutic Approach for Sensitizing Acute Promyelocytic Leukemia Cells to All-Trans Retinoic Acid. Cells, 10."
},
{
"DOI": "10.1016/j.neubiorev.2020.02.013",
"article-title": "Retinoic acid and depressive disorders: Evidence and possible neurobiological mechanisms",
"author": "Hu",
"doi-asserted-by": "crossref",
"first-page": "376",
"journal-title": "Neurosci. Biobehav. Rev.",
"key": "ref_55",
"volume": "112",
"year": "2020"
},
{
"DOI": "10.4088/JCP.10r05993",
"article-title": "Retinoic acid and affective disorders: The evidence for an association",
"author": "Bremner",
"doi-asserted-by": "crossref",
"first-page": "37",
"journal-title": "J. Clin. Psychiatry",
"key": "ref_56",
"volume": "73",
"year": "2012"
},
{
"DOI": "10.3390/ijms22094307",
"doi-asserted-by": "crossref",
"key": "ref_57",
"unstructured": "Salaciak, K., Koszalka, A., Zmudzka, E., and Pytka, K. (2021). The Calcium/Calmodulin-Dependent Kinases II and IV as Therapeutic Targets in Neurodegenerative and Neuropsychiatric Disorders. Int. J. Mol. Sci., 22."
},
{
"DOI": "10.1016/j.nlm.2011.04.017",
"article-title": "Forebrain-specific constitutively active CaMKKalpha transgenic mice show deficits in hippocampus-dependent long-term memory",
"author": "Kaitsuka",
"doi-asserted-by": "crossref",
"first-page": "238",
"journal-title": "Neurobiol. Learn. Mem.",
"key": "ref_58",
"volume": "96",
"year": "2011"
}
],
"reference-count": 58,
"references-count": 58,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1999-4915/16/1/140"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Virology",
"Infectious Diseases"
],
"subtitle": [],
"title": "Retinoic Acid-Mediated Inhibition of Mouse Coronavirus Replication Is Dependent on IRF3 and CaMKK",
"type": "journal-article",
"volume": "16"
}