Hydroxychloroquine for the treatment of severe respiratory infection by COVID-19: a randomized controlled trial
et al., medRxiv, doi:10.1101/2021.02.01.21250371, Feb 2021
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
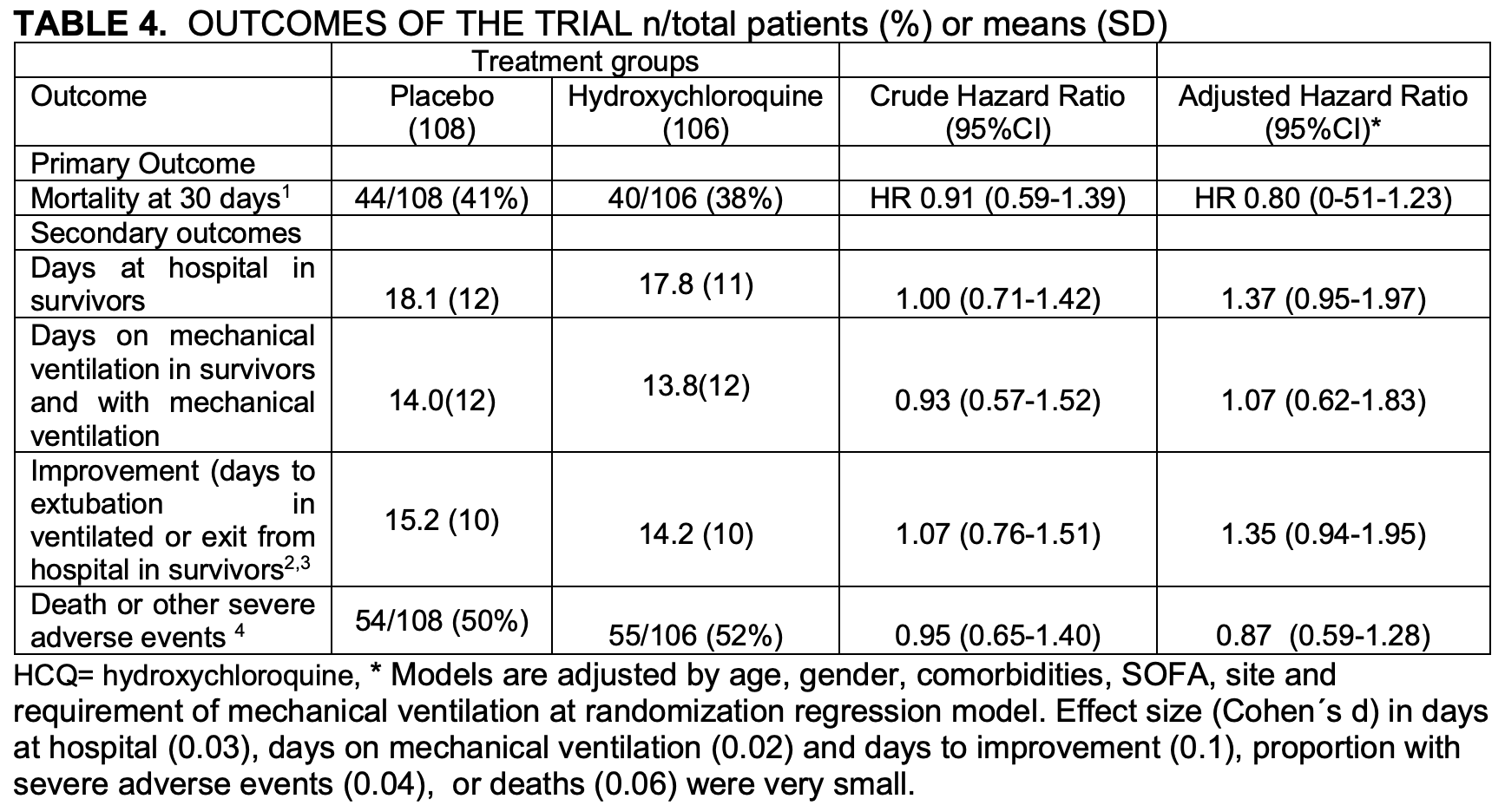
Very late stage RCT with 214 patients, mean SpO2 65%, 162 on mechanical ventilation, showing no significant difference in mortality.
Patients not intubated at baseline show greater improvement, HR 0.43 [0.09-2.03].
Table 4 shows different results to the abstract - table 4 adjusted HR 0.80 [0.51-1.23], abstract HR 0.88 [0.51-1.53]. There was no significant difference in severe adverse events.
|
risk of death, 12.0% lower, RR 0.88, p = 0.66, treatment 106, control 108.
|
|
risk of death, 57.0% lower, RR 0.43, p = 0.29, subgroup not intubated at baseline.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Hernandez-Cardenas et al., 5 Feb 2021, Randomized Controlled Trial, Mexico, preprint, 6 authors, study period 8 April, 2020 - 12 July, 2020, average treatment delay 7.4 days.
HYDROXYCHLOROQUINE FOR THE TREATMENT OF SEVERE RESPIRATORY INFECTION BY COVID-19: A RANDOMIZED CONTROLLED TRIAL
doi:10.1101/2021.02.01.21250371
The novel coronavirus pandemic (COVID-19) represents a major public health problem due to its rapid spread and its ability to generate severe pneumonia. Thus, it is essential to find a treatment that reduces mortality. Our objective was to estimate whether treatment with 400 mg/day of Hydroxychloroquine for 10 days reduces in-hospital mortality in subjects with severe respiratory disease due to COVID-19 compared with placebo.
Material and methods: A double-blind, randomized, placebo-controlled trial to evaluate the safety and efficacy of Hydroxychloroquine for the treatment of severe disease by COVID-19 through an intention-to-treat analysis. Eligible for the study were adults aged more than 18 years with COVID-19 confirmed by RT-PCR and lung injury requiring hospitalization with or without mechanical ventilation. Primary outcome was 30-day mortality. Secondary outcomes: days of mechanical ventilation, days of hospitalization and cumulative incidence of serious adverse events. Results: A total of 214 patients with COVID-19 were recruited, randomized and analyzed. They were hypoxemic with a mean SpO2 of 65% ± 20, tachycardic (pulse rate 108±17 min-1 ) and tachypneic (32 ±10 min-1 ); 162 were under mechanical ventilation at randomization. Thirty-day mortality was similar in both groups (38% in Hydroxychloroquine vs. 41% in placebo, hazard ratio [HR] 0.88, 95% Confidence Interval [95%CI] 0.51-1.53). In the surviving participants, no significant difference was found in secondary outcomes.
Conclusion: No beneficial effect or significant harm could be demonstrated in our randomized controlled trial including 214 patients, using relatively low doses of Hydroxychloroquine compared with placebo in hospitalized patients with severe COVID-19. .
Analysis Follow-Up Randomized (n= 214)
Allocation .
References
Beigel, Tomashek, Dodd, Remdesivir for the Treatment of Covid-19 -Preliminary Report, The New England journal of medicine, doi:10.1056/NEJMoa2007764
Chan, Yuan, Kok, A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster, Lancet
Chen, Zhou, Dong, Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study, Lancet
Holshue, Debolt, Lindquist, First Case of 2019 Novel Coronavirus in the United States, The New England journal of medicine
Horby, Lim, Dexamethasone in Hospitalized Patients with Covid-19 -Preliminary Report, The New England journal of medicine
Marshall, Murthy, Diaz, A minimal common outcome measure set for COVID-19 clinical research, The Lancet Infectious Diseases
Mercuro, Yen, Shim, Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19), JAMA cardiology
Moher, Hopewell, Schulz, Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials, The BMJ
Saitz, Schwitzer, Communicating Science in the Time of a Pandemic, JAMA
Tisdale, Jaynes, Kingery, Development and validation of a risk score to predict QT interval prolongation in hospitalized patients, Circulation. Cardiovascular quality and outcomes
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell research
Zhou, Yang, Wang, A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature
Zhu, Zhang, Wang, A Novel Coronavirus from Patients with Pneumonia in China, 2019, The New England journal of medicine
DOI record:
{
"DOI": "10.1101/2021.02.01.21250371",
"URL": "http://dx.doi.org/10.1101/2021.02.01.21250371",
"abstract": "<jats:title>ABSTRACT</jats:title><jats:p>The novel coronavirus pandemic (COVID–19) represents a major public health problem due to its rapid spread and its ability to generate severe pneumonia. Thus, it is essential to find a treatment that reduces mortality. Our objective was to estimate whether treatment with 400 mg/day of Hydroxychloroquine for 10 days reduces in-hospital mortality in subjects with severe respiratory disease due to COVID-19 compared with placebo.</jats:p><jats:sec><jats:title>Material and methods</jats:title><jats:p>A double-blind, randomized, placebo-controlled trial to evaluate the safety and efficacy of Hydroxychloroquine for the treatment of severe disease by COVID-19 through an intention-to-treat analysis. Eligible for the study were adults aged more than 18 years with COVID-19 confirmed by RT-PCR and lung injury requiring hospitalization with or without mechanical ventilation. Primary outcome was 30-day mortality. Secondary outcomes: days of mechanical ventilation, days of hospitalization and cumulative incidence of serious adverse events.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>A total of 214 patients with COVID-19 were recruited, randomized and analyzed. They were hypoxemic with a mean SpO<jats:sub>2</jats:sub> of 65% ± 20, tachycardic (pulse rate 108±17 min-<jats:sup>1</jats:sup>) and tachypneic (32 ±10 min-<jats:sup>1</jats:sup>); 162 were under mechanical ventilation at randomization. Thirty-day mortality was similar in both groups (38% in Hydroxychloroquine vs. 41% in placebo, hazard ratio [HR] 0.88, 95% Confidence Interval [95%CI] 0.51-1.53). In the surviving participants, no significant difference was found in secondary outcomes.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>No beneficial effect or significant harm could be demonstrated in our randomized controlled trial including 214 patients, using relatively low doses of Hydroxychloroquine compared with placebo in hospitalized patients with severe COVID-19.</jats:p></jats:sec><jats:sec><jats:title>CONSORT GUIDELINES</jats:title><jats:table-wrap id=\"utbl1\" orientation=\"portrait\" position=\"float\"><jats:graphic xmlns:xlink=\"http://www.w3.org/1999/xlink\" xlink:href=\"21250371v1_utbl1\" position=\"float\" orientation=\"portrait\" /></jats:table-wrap></jats:sec>",
"accepted": {
"date-parts": [
[
2021,
2,
5
]
]
},
"author": [
{
"affiliation": [],
"family": "Hernandez-Cardenas",
"given": "Carmen",
"sequence": "first"
},
{
"affiliation": [],
"family": "Thirion-Romero",
"given": "Ireri",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rivera-Martinez",
"given": "Norma E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meza-Meneses",
"given": "Patricia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Remigio-Luna",
"given": "Arantxa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Perez-Padilla",
"given": "Rogelio",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
2,
5
]
],
"date-time": "2021-02-05T21:20:16Z",
"timestamp": 1612560016000
},
"deposited": {
"date-parts": [
[
2021,
12,
15
]
],
"date-time": "2021-12-15T15:46:40Z",
"timestamp": 1639583200000
},
"group-title": "Respiratory Medicine",
"indexed": {
"date-parts": [
[
2024,
3,
1
]
],
"date-time": "2024-03-01T23:36:09Z",
"timestamp": 1709336169354
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 4,
"issued": {
"date-parts": [
[
2021,
2,
5
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.02.01.21250371",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
2,
5
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
2,
5
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30154-9",
"doi-asserted-by": "publisher",
"key": "2021020706551270000_2021.02.01.21250371v1.1"
},
{
"DOI": "10.1016/S0140-6736(20)30211-7",
"doi-asserted-by": "crossref",
"key": "2021020706551270000_2021.02.01.21250371v1.2",
"unstructured": "Chen N , Zhou M , Dong X , et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020."
},
{
"DOI": "10.1056/NEJMoa2001191",
"doi-asserted-by": "publisher",
"key": "2021020706551270000_2021.02.01.21250371v1.3"
},
{
"DOI": "10.1038/s41586-020-2012-7",
"doi-asserted-by": "publisher",
"key": "2021020706551270000_2021.02.01.21250371v1.4"
},
{
"DOI": "10.1056/NEJMoa2001017",
"doi-asserted-by": "publisher",
"key": "2021020706551270000_2021.02.01.21250371v1.5"
},
{
"key": "2021020706551270000_2021.02.01.21250371v1.6",
"unstructured": "World Health Organization. Coronavirus disease (COVID-19) Situation Report– 179. 2020; https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200717-covid-19-sitrep-179.pdf?sfvrsn=2f1599fa_2. Accessed July 18, 2020."
},
{
"DOI": "10.1038/s41422-020-0282-0",
"doi-asserted-by": "publisher",
"key": "2021020706551270000_2021.02.01.21250371v1.7"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "2021020706551270000_2021.02.01.21250371v1.8"
},
{
"key": "2021020706551270000_2021.02.01.21250371v1.9",
"unstructured": "Recovery Collaborative Group, Horby P , Lim WS , et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. The New England journal of medicine. 2020."
},
{
"key": "2021020706551270000_2021.02.01.21250371v1.10",
"unstructured": "Randomised Evaluation of COVid-19 thERapY (RECOVERY) Trial. No clinical benefit from use of hydroxychloroquine in hospitalised patients with COVID-19. 2020; https://www.recoverytrial.net/news/statement-from-the-chief-investigators-of-the-randomised-evaluation-of-covid-19-therapy-recovery-trial-on-hydroxychloroquine-5-june-2020-no-clinical-benefit-from-use-of-hydroxychloroquine-in-hospitalised-patients-with-covid-19."
},
{
"key": "2021020706551270000_2021.02.01.21250371v1.11",
"unstructured": "World Health Organization. WHO discontinues hydroxychloroquine and lopinavir/ritonavir treatment arms for COVID-19. 2020; https://www.who.int/news-room/detail/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19."
},
{
"key": "2021020706551270000_2021.02.01.21250371v1.12",
"unstructured": "National Institutes of Health. NIH halts clinical trial of hydroxychloroquine. 2020; https://www.nih.gov/news-events/news-releases/nih-halts-clinical-trial-hydroxychloroquine. Accessed June 20, 2020."
},
{
"DOI": "10.3736/jcim20100801",
"doi-asserted-by": "crossref",
"key": "2021020706551270000_2021.02.01.21250371v1.13",
"unstructured": "Moher D , Hopewell S , Schulz KF , et al. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. The BMJ. 2010;340."
},
{
"DOI": "10.1001/jamacardio.2020.1834",
"article-title": "Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19)",
"doi-asserted-by": "crossref",
"first-page": "1036",
"issue": "9",
"journal-title": "JAMA cardiology",
"key": "2021020706551270000_2021.02.01.21250371v1.14",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1161/CIRCOUTCOMES.113.000152",
"doi-asserted-by": "publisher",
"key": "2021020706551270000_2021.02.01.21250371v1.15"
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"doi-asserted-by": "publisher",
"key": "2021020706551270000_2021.02.01.21250371v1.16"
},
{
"DOI": "10.1001/jama.2020.12535",
"article-title": "Communicating Science in the Time of a Pandemic",
"doi-asserted-by": "crossref",
"first-page": "443",
"issue": "5",
"journal-title": "JAMA",
"key": "2021020706551270000_2021.02.01.21250371v1.17",
"volume": "324",
"year": "2020"
}
],
"reference-count": 17,
"references-count": 17,
"relation": {
"is-preprint-of": [
{
"asserted-by": "subject",
"id": "10.1371/journal.pone.0257238",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2021.02.01.21250371"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "HYDROXYCHLOROQUINE FOR THE TREATMENT OF SEVERE RESPIRATORY INFECTION BY COVID-19: A RANDOMIZED CONTROLLED TRIAL",
"type": "posted-content"
}
