Azithromycin for treatment of hospitalised COVID-19 patients: a randomised, multicentre, open-label clinical trial (DAWn-AZITHRO)
et al., ERJ Open Research, doi:10.1183/23120541.00610-2021, EudraCT-2020-001614-38A, Jan 2022
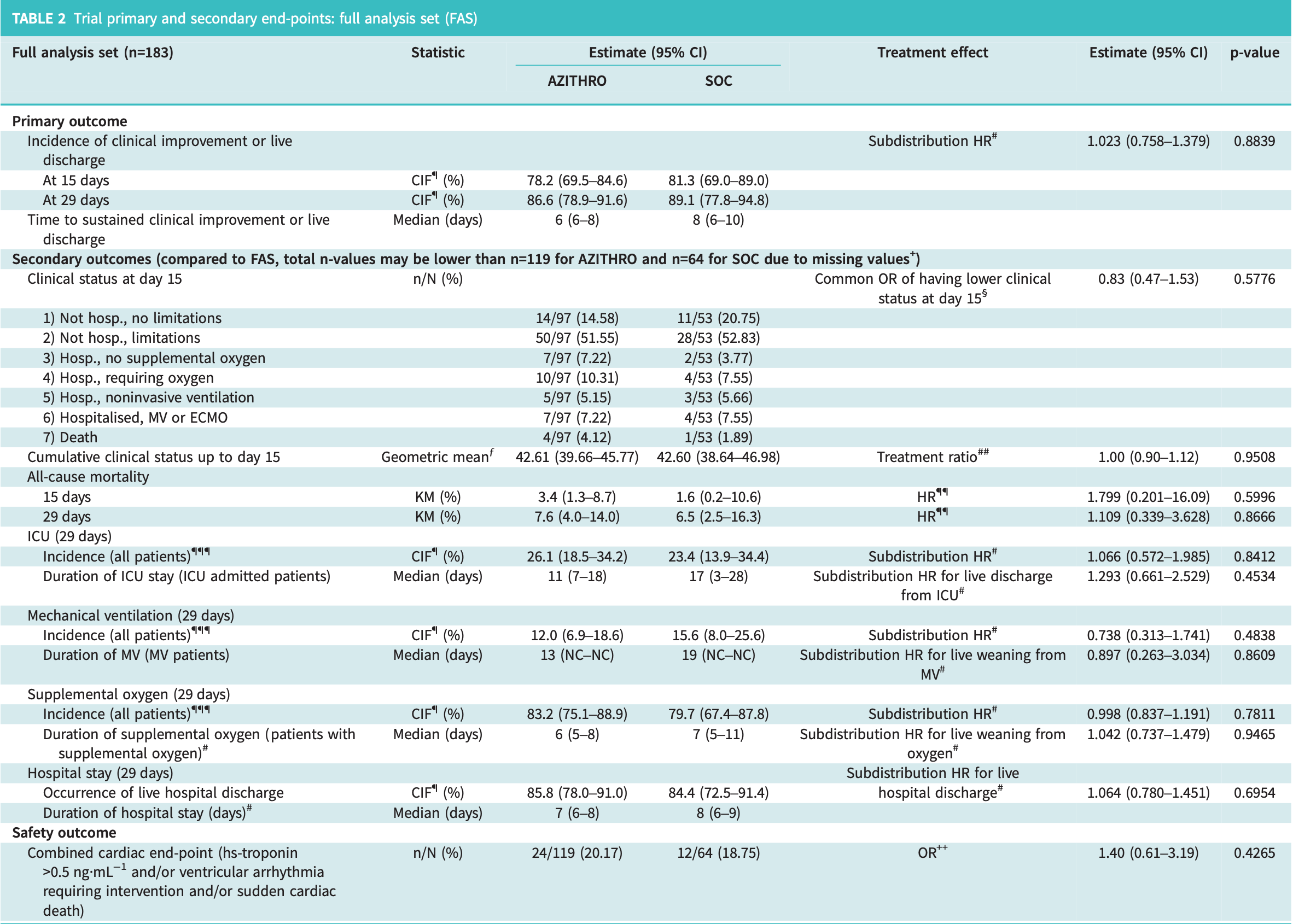
RCT 183 hospitalized COVID-19 patients showing no significant differences with azithromycin treatment.
|
risk of death, 10.9% higher, HR 1.11, p = 0.87, treatment 119, control 64, Kaplan-Meier.
|
|
risk of mechanical ventilation, 26.2% lower, HR 0.74, p = 0.50, treatment 119, control 64.
|
|
risk of ICU admission, 6.6% higher, HR 1.07, p = 0.85, treatment 119, control 64.
|
|
risk of no recovery, 2.2% lower, HR 0.98, p = 0.89, treatment 119, control 64, inverted to make HR<1 favor treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Gyselinck et al., 27 Jan 2022, Randomized Controlled Trial, Belgium, peer-reviewed, mean age 62.0, 23 authors, study period 24 April, 2020 - 17 December, 2021, average treatment delay 7.0 days, trial EudraCT-2020-001614-38A.
Contact: iwein.gyselinck@kuleuven.be.
Azithromycin for treatment of hospitalised COVID-19 patients: a randomised, multicentre, open-label clinical trial (DAWn-AZITHRO)
doi:10.1183/23120541.00610-2021].
Previous randomised controlled studies with azithromycin in hospitalised COVID-19 patients assessed end-points at fixed timepoints. Complementary to this, DAWn-AZITHRO assessed time to sustained improvement. No benefit of azithromycin was shown. https://bit.ly/3FapyC7
References
Abaleke, Abbas, Abbasi, Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet
Butler, Dorward, Yu, Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, Lancet
Cao, Wang, Wen, A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19, N Engl J Med
Cavalcanti, Zampieri, Rosa, Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19, N Engl J Med
Chertow, Memoli, Bacterial coinfection in influenza: a grand rounds review, J Am Med Assoc
Damle, Vourvahis, Wang, Clinical pharmacology perspectives on the antiviral activity of azithromycin and use in COVID-19, Clin Pharmacol Ther, doi:10.1183/23120541.00610-202111
Devos, Van Thillo, Compernolle, Early high antibody titre convalescent plasma for hospitalised COVID-19 patients: DAWn-plasma, Eur Respir J
Dodd, Follmann, Wang, Endpoints for randomized controlled clinical trials for COVID-19 treatments, Clin Trials
Furtado, Berwanger, Fonseca, Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial, Lancet
Gautret, Lagier, Parola, Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study, Travel Med Infect Dis
Gautret, Lagier, Parola, Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial, Int J Antimicrob Agents
Gyselinck, Janssens, Verhamme, Rationale for azithromycin in COVID-19: an overview of existing evidence, BMJ Open Respir Res
Gyselinck, Liesenborgs, Landeloos, Direct antivirals working against the novel coronavirus: azithromycin (DAWn-AZITHRO), a randomized, multicenter, open-label, adaptive, proof-of-concept clinical trial of new antivirals working against SARS-CoV-2azithromycin trial, Trials
Ishaqui, Khan, Sulaiman, Assessment of efficacy of Oseltamivir-Azithromycin combination therapy in prevention of Influenza-A (H1N1)pdm09 infection complications and rapidity of symptoms relief, Expert Rev Respir Med
Lansbury, Lim, Baskaran, Co-infections in people with COVID-19: a systematic review and meta-analysis, J Infect
Lee, Wong, Chan, Anti-inflammatory effects of adjunctive macrolide treatment in adults hospitalized with influenza: a randomized controlled trial, Antiviral Res
Liesenborghs, Spriet, Jochmans, Itraconazole for COVID-19: preclinical studies and a proof-of-concept randomized clinical trial, EBioMedicine
Omrani, Pathan, Thomas, Randomized double-blinded placebo-controlled trial of hydroxychloroquine with or without azithromycin for virologic cure of non-severe Covid-19, EClinicalMedicine
Parnham, Haber, Ej, Azithromycin: mechanisms of action and their relevance for clinical applications, Pharmacol Ther
Rubin, Multiple imputation after 18+ years, J Am Stat Assoc
Sekhavati, Jafari, Seyedalinaghi, Safety and effectiveness of azithromycin in patients with COVID-19: an open-label randomised trial, Int J Antimicrob Agents
Touret, Gilles, Barral, In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication, Sci Rep
Vanassche, Engelen, Van Thillo, A randomized, open-label, adaptive, proof-of-concept clinical trial of modulation of host thromboinflammatory response in patients with COVID-19: the DAWn-Antico study, Trials
Venditto, Haydar, Latif, Immunomodulatory effects of azithromycin revisited: potential applications to COVID-19, Front Immunol
Wang, Zhang, Du, Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial, Lancet, doi:10.1183/23120541.00610-2021
DOI record:
{
"DOI": "10.1183/23120541.00610-2021",
"ISSN": [
"2312-0541"
],
"URL": "http://dx.doi.org/10.1183/23120541.00610-2021",
"abstract": "<jats:sec>\n<jats:title>Background and objectives</jats:title>\n<jats:p>Azithromycin was rapidly adopted as a repurposed drug to treat coronavirus disease 2019 (COVID-19) early in the pandemic. We aimed to evaluate its efficacy in patients hospitalised for COVID-19.</jats:p>\n</jats:sec>\n<jats:sec>\n<jats:title>Methods</jats:title>\n<jats:p>In a series of randomised, open-label, phase 2 proof-of-concept, multicentre clinical trials (Direct Antivirals Working against the novel coronavirus (DAWn)), several treatments were compared with standard of care. In 15 Belgian hospitals, patients hospitalised with moderate to severe COVID-19 were allocated 2:1 to receive standard of care plus azithromycin or standard of care alone. The primary outcome was time to live discharge or sustained clinical improvement, defined as a two-point improvement on the World Health Organization (WHO) ordinal scale sustained for at least 3 days.</jats:p>\n</jats:sec>\n<jats:sec>\n<jats:title>Results</jats:title>\n<jats:p>Patients were included between April 22 and December 17, 2020. When 15-day follow-up data were available for 160 patients (56% of preset cohort), an interim analysis was performed at request of the independent Data Safety and Monitoring Board. Subsequently, DAWn-AZITHRO was stopped for futility. In total, 121 patients were allocated to the treatment arm and 64 patients to the standard-of-care arm. We found no effect of azithromycin on the primary outcome with a hazard ratio of 1.044 (95% CI 0.772–1.413; p=0.7798). None of the predefined subgroups showed significant interaction as covariates in the Fine–Gray regression analysis. No benefit of azithromycin was found on any of the short- and longer-term secondary outcomes.</jats:p>\n</jats:sec>\n<jats:sec>\n<jats:title>Conclusion</jats:title>\n<jats:p>Time to clinical improvement is not influenced by azithromycin in patients hospitalised with moderate to severe COVID-19.</jats:p>\n</jats:sec>",
"accepted": {
"date-parts": [
[
2021,
12,
28
]
]
},
"alternative-id": [
"10.1183/23120541.00610-2021"
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-4068-7228",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gyselinck",
"given": "Iwein",
"sequence": "first"
},
{
"affiliation": [],
"family": "Liesenborghs",
"given": "Laurens",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Belmans",
"given": "Ann",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Engelen",
"given": "Matthias M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Betrains",
"given": "Albrecht",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-3260-280X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Van Thillo",
"given": "Quentin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nguyen",
"given": "Pham Anh Hong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goeminne",
"given": "Pieter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Soenen",
"given": "Ann-Catherine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Maeyer",
"given": "Nikolaas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pilette",
"given": "Charles",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Papleux",
"given": "Emmanuelle",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-5552-1645",
"affiliation": [],
"authenticated-orcid": false,
"family": "Vanderhelst",
"given": "Eef",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Derweduwen",
"given": "Aurélie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alexander",
"given": "Patrick",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-4363-1024",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bouckaert",
"given": "Bernard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martinot",
"given": "Jean-Benoît",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Decoster",
"given": "Lynn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vandeurzen",
"given": "Kurt",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schildermans",
"given": "Rob",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-8698-2858",
"affiliation": [],
"authenticated-orcid": false,
"family": "Verhamme",
"given": "Peter",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-1830-2982",
"affiliation": [],
"authenticated-orcid": false,
"family": "Janssens",
"given": "Wim",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-3468-9251",
"affiliation": [],
"authenticated-orcid": false,
"family": "Vos",
"given": "Robin",
"sequence": "additional"
}
],
"container-title": "ERJ Open Research",
"container-title-short": "ERJ Open Res",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"publications.ersnet.org"
]
},
"created": {
"date-parts": [
[
2022,
1,
27
]
],
"date-time": "2022-01-27T13:30:19Z",
"timestamp": 1643290219000
},
"deposited": {
"date-parts": [
[
2025,
2,
24
]
],
"date-time": "2025-02-24T05:13:56Z",
"timestamp": 1740374036000
},
"funder": [
{
"name": "COVID-19 fund of UZ and KU Leuven"
}
],
"indexed": {
"date-parts": [
[
2025,
2,
25
]
],
"date-time": "2025-02-25T05:09:00Z",
"timestamp": 1740460140230,
"version": "3.37.3"
},
"is-referenced-by-count": 6,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
1
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022,
2,
28
]
]
},
"published-print": {
"date-parts": [
[
2022,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
1
]
],
"date-time": "2022-01-01T00:00:00Z",
"timestamp": 1640995200000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1183/23120541.00610-2021",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "81",
"original-title": [],
"page": "00610-2021",
"prefix": "10.1183",
"published": {
"date-parts": [
[
2022,
1
]
]
},
"published-online": {
"date-parts": [
[
2022,
1,
27
]
]
},
"published-print": {
"date-parts": [
[
2022,
1
]
]
},
"publisher": "European Respiratory Society (ERS)",
"reference": [
{
"DOI": "10.1016/j.ebiom.2021.103288",
"article-title": "Itraconazole for COVID-19: preclinical studies and a proof-of-concept randomized clinical trial",
"author": "Liesenborghs",
"doi-asserted-by": "crossref",
"first-page": "103288",
"journal-title": "EBioM",
"key": "2024101709221868000_8.1.00610-2021.1",
"volume": "66",
"year": "2021"
},
{
"DOI": "10.1186/s13063-021-05033-x",
"article-title": "Direct antivirals working against the novel coronavirus: azithromycin (DAWn-AZITHRO), a randomized, multicenter, open-label, adaptive, proof-of-concept clinical trial of new antivirals working against SARS-CoV-2 – azithromycin trial",
"author": "Gyselinck",
"doi-asserted-by": "crossref",
"first-page": "126",
"journal-title": "Trials",
"key": "2024101709221868000_8.1.00610-2021.2",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1186/s13063-020-04878-y",
"article-title": "A randomized, open-label, adaptive, proof-of-concept clinical trial of modulation of host thromboinflammatory response in patients with COVID-19: the DAWn-Antico study",
"author": "Vanassche",
"doi-asserted-by": "crossref",
"first-page": "1005",
"journal-title": "Trials",
"key": "2024101709221868000_8.1.00610-2021.3",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1183/13993003.01724-2021",
"doi-asserted-by": "crossref",
"key": "2024101709221868000_8.1.00610-2021.4",
"unstructured": "Devos T , Van Thillo Q , Compernolle V , et al. Early high antibody titre convalescent plasma for hospitalised COVID-19 patients: DAWn-plasma. Eur Respir J 2022; 59: 2101724. doi:10.1183/13993003.01724-2021"
},
{
"DOI": "10.1038/s41598-020-70143-6",
"doi-asserted-by": "crossref",
"key": "2024101709221868000_8.1.00610-2021.5",
"unstructured": "Touret F , Gilles M , Barral K , et al. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci Rep 2020; 10: 13093."
},
{
"DOI": "10.1002/cpt.1857",
"doi-asserted-by": "publisher",
"key": "2024101709221868000_8.1.00610-2021.6"
},
{
"DOI": "10.1080/17476348.2020.1730180",
"article-title": "Assessment of efficacy of Oseltamivir-Azithromycin combination therapy in prevention of Influenza-A (H1N1)pdm09 infection complications and rapidity of symptoms relief",
"author": "Ishaqui",
"doi-asserted-by": "crossref",
"first-page": "533",
"journal-title": "Expert Rev Respir Med",
"key": "2024101709221868000_8.1.00610-2021.7",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2017.05.008",
"doi-asserted-by": "publisher",
"key": "2024101709221868000_8.1.00610-2021.8"
},
{
"DOI": "10.1016/jijantimicag2020105949",
"doi-asserted-by": "publisher",
"key": "2024101709221868000_8.1.00610-2021.9"
},
{
"DOI": "10.1016/j.tmaid.2020.101663",
"doi-asserted-by": "publisher",
"key": "2024101709221868000_8.1.00610-2021.10"
},
{
"DOI": "10.1136/bmjresp-2020-000806",
"doi-asserted-by": "publisher",
"key": "2024101709221868000_8.1.00610-2021.11"
},
{
"DOI": "10.1056/NEJMoa2019014",
"article-title": "Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19",
"author": "Cavalcanti",
"doi-asserted-by": "crossref",
"first-page": "2041",
"journal-title": "N Engl J Med",
"key": "2024101709221868000_8.1.00610-2021.12",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31862-6",
"doi-asserted-by": "publisher",
"key": "2024101709221868000_8.1.00610-2021.13"
},
{
"DOI": "10.1016/s0140-6736(21)00149-5",
"doi-asserted-by": "publisher",
"key": "2024101709221868000_8.1.00610-2021.14"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106143",
"doi-asserted-by": "publisher",
"key": "2024101709221868000_8.1.00610-2021.15"
},
{
"DOI": "10.1056/NEJMc2008043",
"doi-asserted-by": "crossref",
"key": "2024101709221868000_8.1.00610-2021.16",
"unstructured": "Cao B , Wang Y , Wen D , et al. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020; 382: 1787–1799."
},
{
"DOI": "10.2307/2291635",
"doi-asserted-by": "publisher",
"key": "2024101709221868000_8.1.00610-2021.17"
},
{
"DOI": "10.1177/1740774520939938",
"article-title": "Endpoints for randomized controlled clinical trials for COVID-19 treatments",
"author": "Dodd",
"doi-asserted-by": "crossref",
"first-page": "472",
"journal-title": "Clin Trials",
"key": "2024101709221868000_8.1.00610-2021.18",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2021.574425",
"article-title": "Immunomodulatory effects of azithromycin revisited: potential applications to COVID-19",
"author": "Venditto",
"doi-asserted-by": "crossref",
"first-page": "285",
"journal-title": "Front Immunol",
"key": "2024101709221868000_8.1.00610-2021.19",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2020.100645",
"article-title": "Randomized double-blinded placebo-controlled trial of hydroxychloroquine with or without azithromycin for virologic cure of non-severe Covid-19",
"author": "Omrani",
"doi-asserted-by": "crossref",
"first-page": "100645",
"journal-title": "EClinicalMedicine",
"key": "2024101709221868000_8.1.00610-2021.20",
"volume": "29",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2020.05.046",
"article-title": "Co-infections in people with COVID-19: a systematic review and meta-analysis",
"author": "Lansbury",
"doi-asserted-by": "crossref",
"first-page": "266",
"journal-title": "J Infect",
"key": "2024101709221868000_8.1.00610-2021.21",
"volume": "81",
"year": "2020"
},
{
"DOI": "10.1001/jama.2012.194139",
"doi-asserted-by": "publisher",
"key": "2024101709221868000_8.1.00610-2021.22"
},
{
"DOI": "10.1016/j.pharmthera.2014.03.003",
"doi-asserted-by": "publisher",
"key": "2024101709221868000_8.1.00610-2021.23"
},
{
"DOI": "10.1016/s0140-6736(21)00461-x",
"doi-asserted-by": "publisher",
"key": "2024101709221868000_8.1.00610-2021.24"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"doi-asserted-by": "publisher",
"key": "2024101709221868000_8.1.00610-2021.25"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "https://publications.ersnet.org/lookup/doi/10.1183/23120541.00610-2021"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Azithromycin for treatment of hospitalised COVID-19 patients: a randomised, multicentre, open-label clinical trial (DAWn-AZITHRO)",
"type": "journal-article",
"update-policy": "https://doi.org/10.1183/ers-crossmark-policy",
"volume": "8"
}
gyselinck