A Cetylpyridinium Chloride Oral Rinse Reduces Salivary Viral Load in Randomized Controlled Trials
et al., JDR Clinical & Translational Research, doi:10.1177/23800844241296840, NCT04584684, Dec 2024
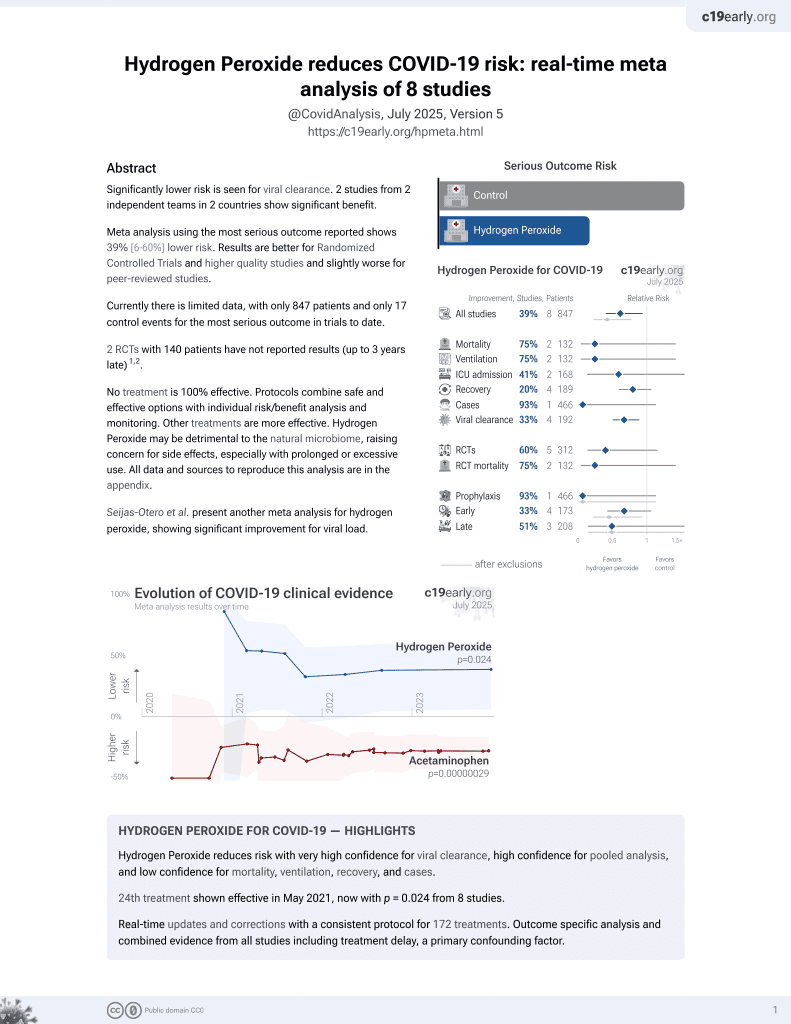
24th treatment shown to reduce risk in
May 2021, now with p = 0.024 from 8 studies.
Lower risk for viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Two RCTs with a total of 247 recently diagnosed COVID-19 patients showing a significant reduction in salivary SARS-CoV-2 viral load 30 minutes after rinsing with a cetylpyridinium chloride (CPC) mouthwash compared to rinsing with saline or water. No significant difference was seen 60 minutes post-rinse or with other mouthwashes. Supplementary tables 9 and 10 show that viral load was lower for all treatments at 60 minutes (including saline and water), without statistical significance. Authors only report short-term viral load, no clinical or longer term results are reported. Patients were late stage, 6-7 days post symptoms, when there has likely been significant viral spread to other tissues.
Analysis of short-term changes in viral load using PCR may not detect
effective treatments because PCR is unable to differentiate between intact
infectious virus and non-infectious or destroyed virus particles. For example
Tarragó-Gil, Alemany perform RCTs with cetylpyridinium chloride
(CPC) mouthwash that show no difference in PCR viral load, however there was
significantly increased detection of SARS-CoV-2 nucleocapsid protein,
indicating viral lysis. CPC inactivates SARS-CoV-2 by degrading its membrane,
exposing the nucleocapsid of the virus. To better estimate changes in viral
load and infectivity, methods like viral culture that can
differentiate intact vs. degraded virus are preferred.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments3.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This study is excluded in meta-analysis:
study only provides short-term viral load results.
|
viral load, 4.5% lower, relative load 0.96, p = 0.98, treatment 17, control 17, N1/N2 combined.
|
|
viral load, 17.3% lower, relative load 0.83, p = 0.96, treatment mean 706.27 (±5785.72) n=17, control mean 854.06 (±10458.7) n=17, N1, 60 min vs. baseline, transformed from log to original scale.
|
|
viral load, 2.0% lower, relative load 0.98, p = 1.00, treatment mean 464.05 (±6944.73) n=17, control mean 473.43 (±10576.1) n=17, N2, 60 min vs. baseline, transformed from log to original scale.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Tarragó-Gil et al., Randomized clinical trial to assess the impact of oral intervention with cetylpyridinium chloride to reduce salivary SARS‐CoV‐2 viral load, Journal of Clinical Periodontology, doi:10.1111/jcpe.13746.
Graves et al., 9 Dec 2024, Double Blind Randomized Controlled Trial, USA, peer-reviewed, mean age 36.0, 16 authors, average treatment delay 6.0 days, trial NCT04584684 (history).
A Cetylpyridinium Chloride Oral Rinse Reduces Salivary Viral Load in Randomized Controlled Trials
doi:10.1177/23800844241296840.
Knowledge Transfer
Author Contributions C. Graves, L.A. Jacox, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; N. Ghaltakhchyan, contributed to data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; T.Q. Ngo, E. Babikow, C. Bocklage, contributed to data acquisition, analysis, and interpretation, and critically revised the manuscript; C. Liu, A. Shoji, M. Freire, K. Divaris, D. Wu, contributed to data analysis and interpretation, critically revised the manuscript; and critically revised Y. Sang, contributed to data acquisition, and critically revised the manuscript; S.T. Phillips, contributed to data acquisition and analysis, and critically revised the manuscript; N. Bowman, S. Wallet, contributed to conception, design, and critically revised the manuscript; S. Frazier-Bowers, contributed to data interpretation, and critically revised the manuscript. All authors gave their final approval and agree to be accountable for all aspects of the work.
Declaration of Conflicting Interests The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
References
Alemany, Perez-Zsolt, Raïch-Regué, Muñoz-Basagoiti, Ouchi et al., Cetylpyridinium chloride mouthwash to reduce shedding of infectious SARS-CoV-2: a double-blind randomized clinical trial, J Dent Res, doi:10.1177/00220345221102310
Alzahrani, Bamashmous, Alkharobi, Alghamdi, Alharbi et al., Mouth rinses efficacy on salivary SARS-CoV-2 viral load: a randomized clinical trial, J Med Virol, doi:10.1002/jmv.28412
Anderson, Patterson, Richards, Pitol, Edwards et al., CPC-containing oral rinses inactivate SARS-CoV-2 variants and are active in the presence of human saliva, J Med Microbiol
Araujo, Estrich, Mikkelsen, Morrissey, Harrison et al., COVID-19 among dentists in the United States, J Am Dent Assoc, doi:10.1016/j.adaj.2021.03.021
Bañó-Polo, Martínez-Gil, Del Pino, Massoli, Mingarro et al., Cetylpyridinium chloride promotes disaggregation of SARS-CoV-2 virus-like particles, J Oral Microbiol, doi:10.1080/20002297.2022.2030094
Bezinelli, Corrêa, Beyerstedt, Franco, Rangel et al., Reduction of SARS-CoV-2 viral load in saliva after rinsing with mouthwashes containing cetylpyridinium chloride: a randomized clinical study, PeerJ, doi:10.7717/peerj.15080
Bidra, Pelletier, Westover, Frank, Brown et al., Comparison of in vitro inactivation of SARS CoV-2 with hydrogen peroxide and povidoneiodine oral antiseptic rinses, J Prosthodont, doi:10.1111/jopr.13220
Bonn, Rohrhofer, Audebert, Lang, Auer et al., Efficacy of a mouthwash containing CHX and CPC in SARS-CoV-2-positive patients: a randomized controlled clinical trial, J Dent Res, doi:10.1177/00220345231156415
Carrouel, Gonçalves, Conte, Campus, Fisher et al., Antiviral activity of reagents in mouth rinses against SARS-CoV-2, J Dent Res, doi:10.1177/0022034520967933
Carrouel, Valette, Gadea, Esparcieux, Illes et al., Use of an antiviral mouthwash as a barrier measure in the SARS-CoV-2 transmission in adults with asymptomatic to mild COVID-19: a multicentre, randomized, doubleblind controlled trial, Clin Microbiol Infect, doi:10.1016/j.cmi.2021.05.028
Chen, Zhao, Peng, Li, Deng et al., Detection of SARS-CoV-2 in saliva and characterization of oral symptoms in COVID-19 patients, Cell Prolif, doi:10.1111/cpr.12923
Cieplik, Jakubovics, Preprocedural mouthwashes for reduction of SARS-CoV-2 viral load and infectivity, J Dent Res, doi:10.1177/00220345221110444
D'amico, Moro, Saracino, Marmiere, Cilona et al., Efficacy of cetylpyridinium chloride mouthwash against SARS-CoV-2: a systematic review of randomized controlled trials, Mol Oral Microbiol, doi:10.1111/omi.12408
Doremalen, Bushmaker, Morris, Holbrook, Gamble et al., Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1, N Engl J Med, doi:10.1056/NEJMc2004973
Elzein, Sater, Fakhreddine, Hanna, Feghali et al., In vivo evaluation of the virucidal efficacy of chlorhexidine and povidone-iodine mouthwashes against salivary SARS-CoV-2: a randomized-controlled clinical trial, J Evid Based Dent Pract, doi:10.1016/j.jebdp.2021.101584
Farmaha, James, Frazier, Sahajpal, Mondal et al., Reduction of SARS-CoV-2 salivary viral load with pre-procedural mouth rinses: a randomised, controlled, clinical trial, Br Dent J, doi:10.1038/s41415-023-5741-9
Graves, Babikow, Ghaltakhchyan, Ngo, Liu et al., Immune dysregulation in the oral cavity during early SARS-CoV-2 infection, J Dent Res, doi:10.1177/00220345241271943
Harrel, Molinari, Aerosols and splatter in dentistry, J Am Dent Assoc, doi:10.14219/jada.archive.2004.0207
Huang, Pérez, Kato, Mikami, Okuda et al., SARS-CoV-2 infection of the oral cavity and saliva, Nat Med, doi:10.1038/s41591-021-01296-8
Iorgulescu, Saliva between normal and pathological: important factors in determining systemic and oral health, J Med Life
Jones, Biele, Mühlemann, Veith, Schneider et al., Estimating infectiousness throughout SARS-CoV-2 infection course, Science, doi:10.1126/science.abi5273
Koch-Heier, Hoffmann, Schindler, Lussi, Planz, Inactivation of SARS-CoV-2 through treatment with the mouth rinsing solutions ViruProX and BacterX Pro, Microorganisms, doi:10.3390/microorganisms9030521
Lamas, Dios, Rodríguez, Campo Pérez, Alvargonzalez et al., Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests, Oral Dis, doi:10.1111/odi.13526
Lazarus, Ratzan, Palayew, Gostin, Larson et al., A global survey of potential acceptance of a COVID-19 vaccine, Nat Med, doi:10.1038/s41591-020-1124-9
Liu, Gallo, Babikow, Wiesen, Jackson et al., Effects of the COVID-19 pandemic on dentists' workforce confidence and workflow, J Am Dent Assoc, doi:10.1016/j.adaj.2021.11.011
Mathieu, Ritchie, Ortiz-Ospina, Roser, Hasell et al., A global database of COVID-19 vaccinations, Nat Hum Behav, doi:10.1038/s41562-021-01122-8
Meister, Brüggemann, Todt, Conzelmann, Müller et al., Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2, J Infect Dis, doi:10.1093/infdis/jiaa471
Meyers, Robison, Milici, Alam, Quillen et al., Lowering the transmission and spread of human coronavirus, J Med Virol, doi:10.1002/jmv.26514
Mohd-Said, Tn, Suhaimi, Rani, Mcgrath, Effectiveness of pre-procedural mouth rinses in reducing aerosol contamination during periodontal prophylaxis: a systematic review, Front Med (Lausanne), doi:10.3389/fmed.2021.600769
Muñoz-Basagoiti, Perez-Zsolt, León, Blanc, Raïch-Regué et al., Mouthwashes with CPC reduce the infectivity of SARS-CoV-2 variants in vitro, J Dent Res, doi:10.1177/00220345211029269
Naqvi, Citardi, Cattano, Ostrosky-Zeichner, Knackstedt et al., Povidone-iodine solution as SARS-CoV-2 prophylaxis for procedures of the upper aerodigestive tract a theoretical framework, J Otolaryngol Head Neck Surg, doi:10.1186/s40463-020-00474-x
O'donnell, Thomas, Stanton, Maillard, Murphy et al., Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection, Function, doi:10.1093/function/zqaa002
Okamoto, Saito, Okabayashi, Komine, Virucidal activity and mechanism of action of cetylpyridinium chloride against SARS-CoV-2, J Oral Maxillofac Surg Med Pathol, doi:10.1016/j.ajoms.2022.04.001
Panico, Bagordo, Nolasco, Grassi, Bianco et al., Kinetics of SARS-CoV-2 viral load in hospitalized patients, Pathogens, doi:10.3390/pathogens13050429
Perussolo, Teh, Gkranias, Tiberi, Petrie et al., Efficacy of three antimicrobial mouthwashes in reducing SARS-CoV-2 viral load in the saliva of hospitalized patients: a randomized controlled pilot study, Sci Rep, doi:10.1038/s41598-023-39308-x
Platten, Hoffmann, Grosser, Wisplinghoff, Wisplinghoff et al., SARS-CoV-2, CT-values, and infectivity-conclusions to be drawn from side observations, Viruses, doi:10.3390/v13081459
Puhach, Meyer, Eckerle, SARS-CoV-2 viral load and shedding kinetics, Nat Rev Microbiol, doi:10.1038/s41579-022-00822-w
DOI record:
{
"DOI": "10.1177/23800844241296840",
"ISSN": [
"2380-0844",
"2380-0852"
],
"URL": "http://dx.doi.org/10.1177/23800844241296840",
"abstract": "<jats:sec><jats:title>Introduction:</jats:title><jats:p> Evaluating the antiviral potential of commercially available mouthrinses on SARS-CoV-2 holds potential for reducing transmission, particularly as novel variants emerge. Because SARS-CoV-2 is transmitted primarily through salivary and respiratory secretions and aerosols, strategies to reduce salivary viral burden in an antigen-agnostic manner are attractive for mitigating spread in dental, otolaryngology, and orofacial surgery clinics where patients may need to unmask. </jats:p></jats:sec><jats:sec><jats:title>Methods:</jats:title><jats:p> Patients ( n = 128) with confirmed COVID-19–positive status within 10 days of symptom onset or positive test result were enrolled in a double-blind randomized controlled trial of Food and Drug Administration–approved mouthrinses containing active ingredients ethanol, hydrogen peroxide, povidone iodine, chlorhexidine gluconate, cetylpyridinium chloride (CPC), or saline. The CPC, ethanol, and sterile water rinses were followed in a second double-blind randomized controlled trial ( n = 230). Participants provided a saliva sample before rinsing (baseline) and again at 30 and 60 min after rinse. Quantitative polymerase chain reaction was used to determine salivary SARS-CoV-2 viral load at all time points. An adjusted linear mixed-effect model was employed to compare viral load after rinsing relative to baseline. </jats:p></jats:sec><jats:sec><jats:title>Results:</jats:title><jats:p> The rinse containing CPC significantly reduced salivary SARS-CoV-2 viral load 30 min postrinse relative to baseline ( P = .015), whereas no other rinse significantly affected viral load at 30 min after rinsing. At 60 min postrinsing, no group had a significant reduction in SARS-CoV-2 copy number relative to baseline, indicating a rebound in salivary viral load over a 1-hour window. Participants indicated a fair to good rinsing experience with the CPC product and high willingness to use oral rinses before and during dental and medical health care visits. </jats:p></jats:sec><jats:sec><jats:title>Conclusions:</jats:title><jats:p> Our findings suggest that preprocedural oral rinsing could be implemented as a feasible, inexpensive approach to mitigate spread of SARS-CoV-2 and potentially other enveloped viruses for short periods, which is relevant to clinical procedures involving the nasal and oropharyngeal region. </jats:p></jats:sec><jats:sec><jats:title>Knowledge Transfer Statement:</jats:title><jats:p> Rinsing with a cetylpyridinium chloride–containing mouthrinse can significantly reduce salivary SARS-CoV-2 viral load for up to 30 min; patients are willing to use mouthrinses in medical and dental settings to limit transmission risk in clinics. </jats:p></jats:sec>",
"alternative-id": [
"10.1177/23800844241296840"
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-7387-2462",
"affiliation": [
{
"name": "Department of Biomedical Sciences, Adams School of Dentistry, University of North Carolina, Chapel Hill, NC, USA"
}
],
"authenticated-orcid": false,
"family": "Graves",
"given": "C.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Biomedical Sciences, Adams School of Dentistry, University of North Carolina, Chapel Hill, NC, USA"
},
{
"name": "Department of Orthodontics, Adams School of Dentistry, University of North Carolina, Chapel Hill, NC, USA"
}
],
"family": "Ghaltakhchyan",
"given": "N.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biomedical Sciences, Adams School of Dentistry, University of North Carolina, Chapel Hill, NC, USA"
},
{
"name": "Department of Orthodontics, Adams School of Dentistry, University of North Carolina, Chapel Hill, NC, USA"
}
],
"family": "Ngo",
"given": "T.Q.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics, University of North Carolina, Chapel Hill, NC, USA"
}
],
"family": "Liu",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Orthodontics, Adams School of Dentistry, University of North Carolina, Chapel Hill, NC, USA"
}
],
"family": "Babikow",
"given": "E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biomedical Sciences, Adams School of Dentistry, University of North Carolina, Chapel Hill, NC, USA"
},
{
"name": "Department of Orthodontics, Adams School of Dentistry, University of North Carolina, Chapel Hill, NC, USA"
}
],
"family": "Shoji",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Orthodontics, Adams School of Dentistry, University of North Carolina, Chapel Hill, NC, USA"
}
],
"family": "Bocklage",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biomedical Sciences, Adams School of Dentistry, University of North Carolina, Chapel Hill, NC, USA"
}
],
"family": "Sang",
"given": "Y.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics, University of North Carolina, Chapel Hill, NC, USA"
}
],
"family": "Phillips",
"given": "S.T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Medicine, University of North Carolina, Chapel Hill, NC, USA"
}
],
"family": "Bowman",
"given": "N.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Orthodontics, Adams School of Dentistry, University of North Carolina, Chapel Hill, NC, USA"
}
],
"family": "Frazier-Bowers",
"given": "S.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-4906-7698",
"affiliation": [
{
"name": "J. Craig Venter Institute, La Jolla, CA, USA"
}
],
"authenticated-orcid": false,
"family": "Freire",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Oral Biology, College of Dentistry, University of Florida, Gainesville, FL, USA"
}
],
"family": "Wallet",
"given": "S.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-1290-7251",
"affiliation": [
{
"name": "Department of Pediatric Dentistry and Dental Public Health, Adams School of Dentistry, University of North Carolina, Chapel Hill, NC, USA"
},
{
"name": "Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, CA, USA. Present address for E.B.: Parrott Orthodontics, Staunton, VA, USA. Present address for S.F.B.: School of Dentistry, Indiana University, Indianapolis, Indiana, USA"
}
],
"authenticated-orcid": false,
"family": "Divaris",
"given": "K.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics, University of North Carolina, Chapel Hill, NC, USA"
}
],
"family": "Wu",
"given": "D.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-1923-6825",
"affiliation": [
{
"name": "Department of Biomedical Sciences, Adams School of Dentistry, University of North Carolina, Chapel Hill, NC, USA"
},
{
"name": "Department of Orthodontics, Adams School of Dentistry, University of North Carolina, Chapel Hill, NC, USA"
}
],
"authenticated-orcid": false,
"family": "Jacox",
"given": "L.A.",
"sequence": "additional"
}
],
"container-title": "JDR Clinical & Translational Research",
"container-title-short": "JDR Clinical & Translational Research",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.sagepub.com"
]
},
"created": {
"date-parts": [
[
2024,
12,
9
]
],
"date-time": "2024-12-09T11:44:53Z",
"timestamp": 1733744693000
},
"deposited": {
"date-parts": [
[
2024,
12,
9
]
],
"date-time": "2024-12-09T11:45:07Z",
"timestamp": 1733744707000
},
"funder": [
{
"name": "American Association of Orthodontists Foundation's Robert L. Boyd Biomedical Research Award"
},
{
"name": "American Association of Orthodontists Foundation (AAOF) Resident Research Aid Award"
},
{
"name": "Southern Association of Orthodontists Research Award"
},
{
"DOI": "10.13039/100000072",
"award": [
"1K08DE030235-01A1"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100000072",
"id-type": "DOI"
}
],
"name": "National Institute of Dental and Craniofacial Research"
},
{
"DOI": "10.13039/100000072",
"award": [
"1R03DE031301-01"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100000072",
"id-type": "DOI"
}
],
"name": "National Institute of Dental and Craniofacial Research"
}
],
"indexed": {
"date-parts": [
[
2024,
12,
10
]
],
"date-time": "2024-12-10T05:12:36Z",
"timestamp": 1733807556545,
"version": "3.30.1"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
12,
9
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
12,
9
]
],
"date-time": "2024-12-09T00:00:00Z",
"timestamp": 1733702400000
}
}
],
"link": [
{
"URL": "https://journals.sagepub.com/doi/pdf/10.1177/23800844241296840",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journals.sagepub.com/doi/full-xml/10.1177/23800844241296840",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journals.sagepub.com/doi/pdf/10.1177/23800844241296840",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "179",
"original-title": [],
"prefix": "10.1177",
"published": {
"date-parts": [
[
2024,
12,
9
]
]
},
"published-online": {
"date-parts": [
[
2024,
12,
9
]
]
},
"publisher": "SAGE Publications",
"reference": [
{
"DOI": "10.1177/00220345221102310",
"doi-asserted-by": "publisher",
"key": "bibr1-23800844241296840"
},
{
"DOI": "10.1002/jmv.28412",
"doi-asserted-by": "publisher",
"key": "bibr2-23800844241296840"
},
{
"DOI": "10.1099/jmm.0.001508",
"doi-asserted-by": "publisher",
"key": "bibr3-23800844241296840"
},
{
"DOI": "10.1016/j.adaj.2021.03.021",
"doi-asserted-by": "publisher",
"key": "bibr4-23800844241296840"
},
{
"DOI": "10.1080/20002297.2022.2030094",
"doi-asserted-by": "publisher",
"key": "bibr5-23800844241296840"
},
{
"DOI": "10.7717/peerj.15080",
"doi-asserted-by": "publisher",
"key": "bibr6-23800844241296840"
},
{
"DOI": "10.1111/jopr.13220",
"doi-asserted-by": "publisher",
"key": "bibr7-23800844241296840"
},
{
"DOI": "10.1177/00220345231156415",
"doi-asserted-by": "publisher",
"key": "bibr8-23800844241296840"
},
{
"DOI": "10.1177/0022034520967933",
"doi-asserted-by": "publisher",
"key": "bibr9-23800844241296840"
},
{
"DOI": "10.1016/j.cmi.2021.05.028",
"doi-asserted-by": "publisher",
"key": "bibr10-23800844241296840"
},
{
"DOI": "10.1111/cpr.12923",
"doi-asserted-by": "publisher",
"key": "bibr11-23800844241296840"
},
{
"DOI": "10.1177/00220345221110444",
"doi-asserted-by": "publisher",
"key": "bibr12-23800844241296840"
},
{
"DOI": "10.1111/omi.12408",
"doi-asserted-by": "publisher",
"key": "bibr13-23800844241296840"
},
{
"DOI": "10.1056/NEJMc2004973",
"doi-asserted-by": "publisher",
"key": "bibr14-23800844241296840"
},
{
"DOI": "10.1016/j.jebdp.2021.101584",
"doi-asserted-by": "publisher",
"key": "bibr15-23800844241296840"
},
{
"DOI": "10.1038/s41415-023-5741-9",
"doi-asserted-by": "publisher",
"key": "bibr16-23800844241296840"
},
{
"DOI": "10.1177/00220345241271943",
"doi-asserted-by": "publisher",
"key": "bibr17-23800844241296840"
},
{
"DOI": "10.14219/jada.archive.2004.0207",
"doi-asserted-by": "publisher",
"key": "bibr18-23800844241296840"
},
{
"DOI": "10.1038/s41591-021-01296-8",
"doi-asserted-by": "publisher",
"key": "bibr19-23800844241296840"
},
{
"author": "Iorgulescu G",
"issue": "3",
"journal-title": "J Med Life",
"key": "bibr20-23800844241296840",
"volume": "2",
"year": "2009"
},
{
"key": "bibr21-23800844241296840",
"unstructured": "Johns Hopkins University & Medicine. 2023. COVID-19 map. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html"
},
{
"DOI": "10.1126/science.abi5273",
"doi-asserted-by": "publisher",
"key": "bibr22-23800844241296840"
},
{
"DOI": "10.3390/microorganisms9030521",
"doi-asserted-by": "publisher",
"key": "bibr23-23800844241296840"
},
{
"DOI": "10.1038/s41591-020-1124-9",
"doi-asserted-by": "publisher",
"key": "bibr24-23800844241296840"
},
{
"DOI": "10.1016/j.adaj.2021.11.011",
"doi-asserted-by": "publisher",
"key": "bibr25-23800844241296840"
},
{
"DOI": "10.1111/odi.13526",
"doi-asserted-by": "publisher",
"key": "bibr26-23800844241296840"
},
{
"DOI": "10.1038/s41562-021-01122-8",
"doi-asserted-by": "publisher",
"key": "bibr27-23800844241296840"
},
{
"DOI": "10.1093/infdis/jiaa471",
"doi-asserted-by": "publisher",
"key": "bibr28-23800844241296840"
},
{
"DOI": "10.1002/jmv.26514",
"doi-asserted-by": "publisher",
"key": "bibr29-23800844241296840"
},
{
"DOI": "10.3389/fmed.2021.600769",
"doi-asserted-by": "publisher",
"key": "bibr30-23800844241296840"
},
{
"DOI": "10.1177/00220345211029269",
"doi-asserted-by": "publisher",
"key": "bibr31-23800844241296840"
},
{
"DOI": "10.1186/s40463-020-00474-x",
"doi-asserted-by": "publisher",
"key": "bibr32-23800844241296840"
},
{
"DOI": "10.1093/function/zqaa002",
"doi-asserted-by": "publisher",
"key": "bibr33-23800844241296840"
},
{
"DOI": "10.1016/j.ajoms.2022.04.001",
"doi-asserted-by": "publisher",
"key": "bibr34-23800844241296840"
},
{
"DOI": "10.3390/pathogens13050429",
"doi-asserted-by": "publisher",
"key": "bibr35-23800844241296840"
},
{
"DOI": "10.1038/s41598-023-39308-x",
"doi-asserted-by": "publisher",
"key": "bibr36-23800844241296840"
},
{
"DOI": "10.3390/v13081459",
"doi-asserted-by": "publisher",
"key": "bibr37-23800844241296840"
},
{
"DOI": "10.1038/s41579-022-00822-w",
"doi-asserted-by": "publisher",
"key": "bibr38-23800844241296840"
}
],
"reference-count": 38,
"references-count": 38,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.sagepub.com/doi/10.1177/23800844241296840"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "A Cetylpyridinium Chloride Oral Rinse Reduces Salivary Viral Load in Randomized Controlled Trials",
"type": "journal-article",
"update-policy": "https://doi.org/10.1177/sage-journals-update-policy"
}
graves