Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests
et al., Oral Diseases, doi:10.1111/odi.13526, Jul 2020
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
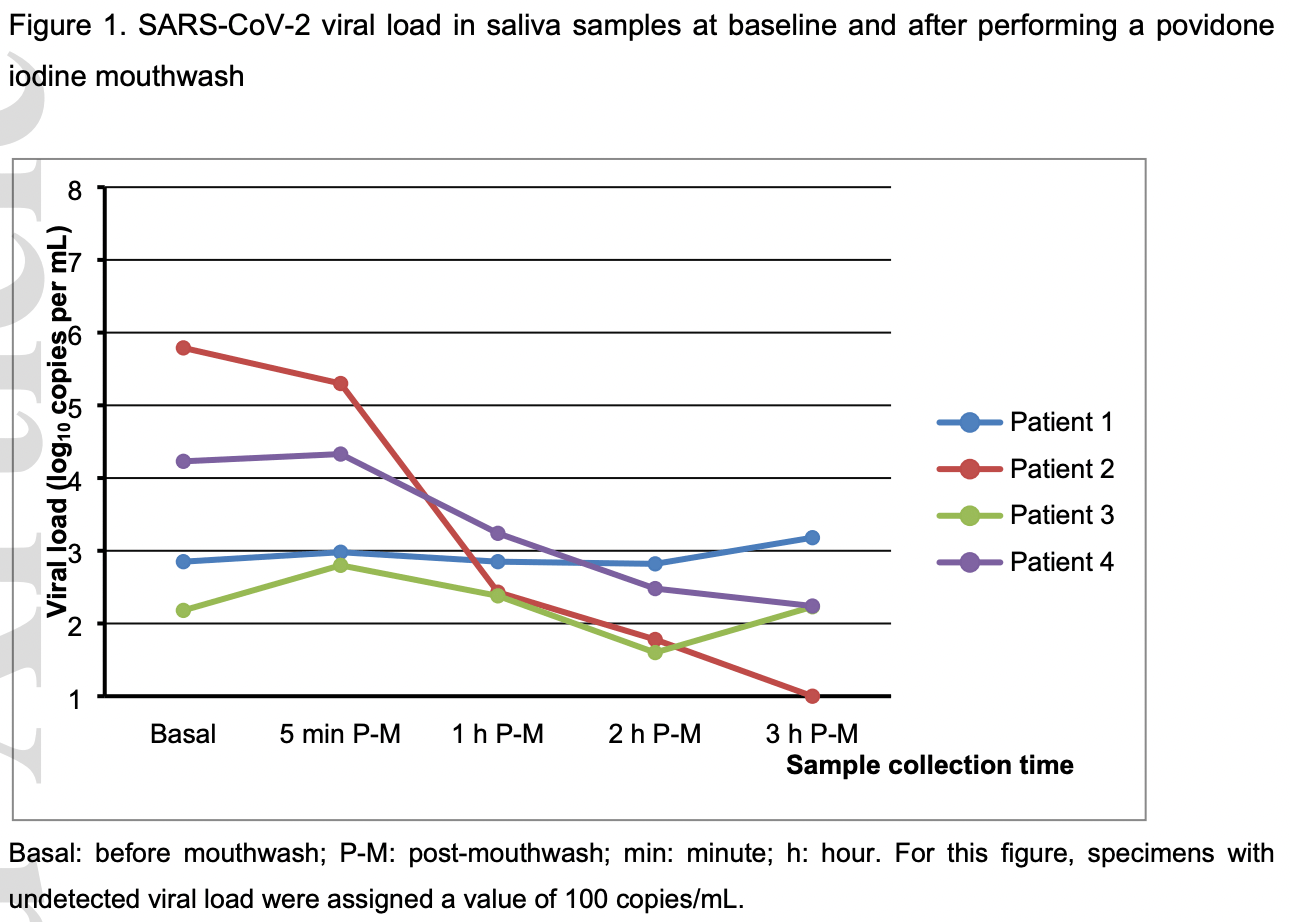
Small study analyzing the impact of PVP-I mouthwash on the salivary viral load of SARS-CoV-2 in 4 patients with COVID-19. In 2 of the 4 patients (those with a higher initial viral load), PVP-I resulted in a significant drop in viral load, which remained after 3 hours.
Martínez Lamas et al., 2 Jul 2020, peer-reviewed, 9 authors.
Abstract: Received: 11 June 2020
|
Revised: 22 June 2020
|
Accepted: 22 June 2020
DOI: 10.1111/odi.13526
S H O R T C O M M U N I C AT I O N
Is povidone iodine mouthwash effective against SARS-CoV-2?
First in vivo tests
Lucía Martínez Lamas1 | Pedro Diz Dios2
| Maria Teresa Pérez Rodríguez3 |
Victor Del Campo Pérez4 | Jorge Julio Cabrera Alvargonzalez1 |
Ana María López Domínguez3 | Javier Fernandez Feijoo2 | Marcio Diniz Freitas2 |
Jacobo Limeres Posse2
1
Department of Microbiology, Microbiology and Infectology Research Group, Galicia Sur Health Research Institute (IIS Galicia Sur) SERGAS-Universidade de
Vigo, Complexo Hospitalario Universitario de Vigo (CHUVI), Sergas, Vigo, Spain
2
Medical-Surgical Dentistry Research Group (OMEQUI), Health Research Institute of Santiago de Compostela (IDIS), University of Santiago de Compostela
(USC), Santiago de Compostela, Spain
3
Internal Medicine Department, Infectious Diseases Unit, Complexo Hospitalario Universitario de Vigo (CHUVI), Galicia Sur Health Research Institute (IIS
Galicia Sur) SERGAS-Universidade de Vigo, Vigo, Spain
4
Preventive Medicine Department, Complexo Hospitalario Universitario de Vigo (CHUVI), Sergas, Vigo, Spain
Correspondence: Pedro Diz Dios, Department of Surgery and Medical-Surgical Specialties, Faculty of Medicine and Odontology, University of Santiago de
Compostela, c/Entrerríos sn, 15782 Santiago de Compostela, Spain.
Email: pedro.diz@usc.es
Keywords: aerosol, coronavirus, COVID-19, mouthwash, povidone iodine, SARS-CoV-2
1 | I NTRO D U C TI O N
transmission of this new coronavirus? To date, the efficacy of povidone against SARS-CoV-2 has not been confirmed. In this study, we
The detection of SARS-CoV-2 in the saliva of patients with coro-
analyzed the impact of a mouthwash with PVP-I on the salivary viral
navirus disease (COVID-19) has made this biological fluid relevant
load of SARS-CoV-2 in 4 patients with COVID-19.
in terms of the diagnosis and transmission of the infection (Azzi,
Carcano, Gianfagna, et al., 2020; To, Tsang, Yip, et al., 2020). As a
result, the dental clinic is considered an environment of risk for dental healthcare personnel and their patients, particularly due to the
potential transmission of the virus through droplets and aerosols
2 | M E TH O DS
2.1 | Patient 1
(Peng et al., 2020). Based on this argument, it has been suggested
that the measures for controlling cross-infection during dental prac-
A 74-year-old man with a history of B-cell non-Hodgkin's lymphoma
tice should include a preprocedural mouth rinse containing oxida-
was admitted to the hospital 28 days ago due to pneumonia by
tive agents such as 1% hydrogen peroxide and 0.2% povidone iodine
SARS-CoV-2. The patient underwent treatment with hydroxychlo-
(PVP-I) (Peng et al., 2020). This protocol, with small variations, has
roquine, lopinavir + ritonavir, corticosteroids, and tocilizumab. The
been accepted by the main professional dental associations world-
patient required admission to the intensive care unit and endotra-
wide, such as the American Dental Association (ADA, 2020). If an
cheal intubation. The patient is currently hospitalized in the hospital
antiseptic mouthwash's virucidal activity is demonstrated, why
ward, without exogenous oxygen supply, is undergoing treatment
limit its application to the dental clinic setting and not adminis-
with rituximab, and presents positive serial polymerase chain reac-
ter it routinely to the entire population to..
DOI record:
{
"DOI": "10.1111/odi.13526",
"ISSN": [
"1354-523X",
"1601-0825"
],
"URL": "http://dx.doi.org/10.1111/odi.13526",
"alternative-id": [
"10.1111/odi.13526"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2020-06-11"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2020-06-22"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2020-07-29"
}
],
"author": [
{
"affiliation": [
{
"name": "Department of Microbiology Microbiology and Infectology Research Group Galicia Sur Health Research Institute (IIS Galicia Sur) SERGAS‐Universidade de Vigo Complexo Hospitalario Universitario de Vigo (CHUVI) Sergas, Vigo Spain"
}
],
"family": "Martínez Lamas",
"given": "Lucía",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-1483-401X",
"affiliation": [
{
"name": "Medical‐Surgical Dentistry Research Group (OMEQUI) Health Research Institute of Santiago de Compostela (IDIS) University of Santiago de Compostela (USC) Santiago de Compostela Spain"
}
],
"authenticated-orcid": false,
"family": "Diz Dios",
"given": "Pedro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internal Medicine Department Infectious Diseases Unit Complexo Hospitalario Universitario de Vigo (CHUVI) Galicia Sur Health Research Institute (IIS Galicia Sur) SERGAS‐Universidade de Vigo Vigo Spain"
}
],
"family": "Pérez Rodríguez",
"given": "Maria Teresa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Preventive Medicine Department Complexo Hospitalario Universitario de Vigo (CHUVI) Sergas, Vigo Spain"
}
],
"family": "Del Campo Pérez",
"given": "Victor",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Microbiology Microbiology and Infectology Research Group Galicia Sur Health Research Institute (IIS Galicia Sur) SERGAS‐Universidade de Vigo Complexo Hospitalario Universitario de Vigo (CHUVI) Sergas, Vigo Spain"
}
],
"family": "Cabrera Alvargonzalez",
"given": "Jorge Julio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internal Medicine Department Infectious Diseases Unit Complexo Hospitalario Universitario de Vigo (CHUVI) Galicia Sur Health Research Institute (IIS Galicia Sur) SERGAS‐Universidade de Vigo Vigo Spain"
}
],
"family": "López Domínguez",
"given": "Ana María",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medical‐Surgical Dentistry Research Group (OMEQUI) Health Research Institute of Santiago de Compostela (IDIS) University of Santiago de Compostela (USC) Santiago de Compostela Spain"
}
],
"family": "Fernandez Feijoo",
"given": "Javier",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medical‐Surgical Dentistry Research Group (OMEQUI) Health Research Institute of Santiago de Compostela (IDIS) University of Santiago de Compostela (USC) Santiago de Compostela Spain"
}
],
"family": "Diniz Freitas",
"given": "Marcio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medical‐Surgical Dentistry Research Group (OMEQUI) Health Research Institute of Santiago de Compostela (IDIS) University of Santiago de Compostela (USC) Santiago de Compostela Spain"
}
],
"family": "Limeres Posse",
"given": "Jacobo",
"sequence": "additional"
}
],
"container-title": "Oral Diseases",
"container-title-short": "Oral Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2020,
7,
2
]
],
"date-time": "2020-07-02T21:09:30Z",
"timestamp": 1593724170000
},
"deposited": {
"date-parts": [
[
2023,
8,
25
]
],
"date-time": "2023-08-25T19:07:02Z",
"timestamp": 1692990422000
},
"indexed": {
"date-parts": [
[
2024,
5,
9
]
],
"date-time": "2024-05-09T21:12:30Z",
"timestamp": 1715289150921
},
"is-referenced-by-count": 68,
"issue": "S1",
"issued": {
"date-parts": [
[
2020,
7,
29
]
]
},
"journal-issue": {
"issue": "S1",
"published-print": {
"date-parts": [
[
2022,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
7,
29
]
],
"date-time": "2020-07-29T00:00:00Z",
"timestamp": 1595980800000
}
}
],
"link": [
{
"URL": "https://api.wiley.com/onlinelibrary/tdm/v1/articles/10.1111%2Fodi.13526",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/odi.13526",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/odi.13526",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/odi.13526",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "908-911",
"prefix": "10.1111",
"published": {
"date-parts": [
[
2020,
7,
29
]
]
},
"published-online": {
"date-parts": [
[
2020,
7,
29
]
]
},
"published-print": {
"date-parts": [
[
2022,
4
]
]
},
"publisher": "Wiley",
"reference": [
{
"key": "e_1_2_7_2_1",
"unstructured": "ADA Interim Guidance for Minimizing Risk of COVID‐19 Transmission.https://www.ada.org/~/media/CPS/Files/COVID/ADA_COVID_Int_Guidance_Treat_Pts.pdf. Accessed May 10 2020"
},
{
"article-title": "Two cases of COVID‐19 with positive salivary and negative pharyngeal or respiratory swabs at hospital discharge: A rising concern",
"author": "Azzi L.",
"first-page": "1",
"journal-title": "Oral Diseases",
"key": "e_1_2_7_3_1",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2020.04.005",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_4_1"
},
{
"DOI": "10.1007/s40121-018-0200-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_5_1"
},
{
"DOI": "10.1159/000089211",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_6_1"
},
{
"article-title": "Guidelines for infection control in dental health‐care settings–2003",
"author": "Kohn W. G.",
"first-page": "1",
"issue": "17",
"journal-title": "MMWR ‐ Recommendations and Reports",
"key": "e_1_2_7_7_1",
"volume": "52",
"year": "2003"
},
{
"DOI": "10.1016/S0140-6736(20)30251-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_8_1"
},
{
"DOI": "10.1093/function/zqaa002",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_9_1"
},
{
"DOI": "10.1038/s41368-020-0075-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_10_1"
},
{
"DOI": "10.1016/S1473-3099(20)30196-1",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_11_1"
},
{
"article-title": "Consistent detection of 2019 novel coronavirus in saliva",
"author": "To K. K.",
"first-page": "1",
"journal-title": "Clinical Infectious Diseases",
"key": "e_1_2_7_12_1",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.05.030",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_13_1"
}
],
"reference-count": 12,
"references-count": 12,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1111/odi.13526"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Is povidone iodine mouthwash effective against SARS‐CoV‐2? First in vivo tests",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "28"
}
