NASAFYTOL® supplementation in adults hospitalized with COVID-19 infection: results from an exploratory open-label randomized controlled trial
et al., Frontiers in Nutrition, doi:10.3389/fnut.2023.1137407, NCT04844658, Jun 2023
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 49 hospitalized COVID-19 patients, 25 treated with curcumin and quercetin, shower lower mortality/ICU admission and improved recovery with treatment. All patients received vitamin D.
336mg curcumin, 520mg quercetin, and 18μg vitamin D3 daily for 14 days. The control arm received 20μg vitamin D3 daily. Baseline fever favored treatment while vaccination favored control.
This is the 19th of 21 COVID-19 RCTs for curcumin, which collectively show efficacy with p=0.0000022.
This is the 25th of 28 COVID-19 controlled studies for curcumin, which collectively show efficacy with p=0.0000000061.
Study covers curcumin and quercetin.
|
risk of death, 67.1% lower, RR 0.33, p = 0.49, treatment 0 of 25 (0.0%), control 1 of 24 (4.2%), NNT 24, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 7.
|
|
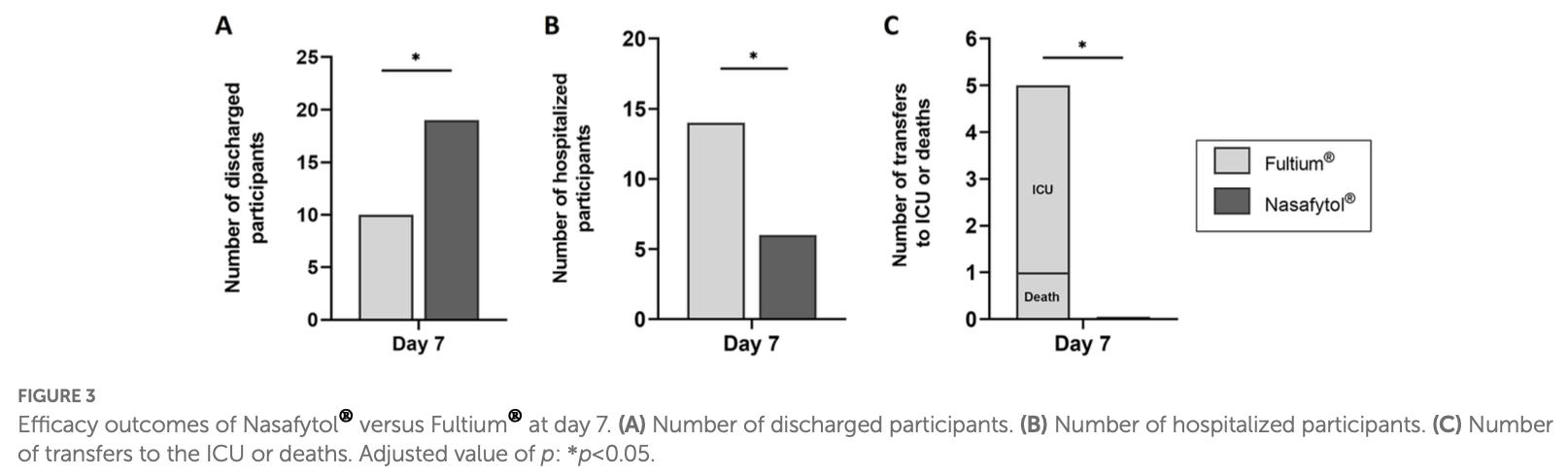
risk of death/ICU, 91.1% lower, RR 0.09, p = 0.02, treatment 0 of 25 (0.0%), control 5 of 24 (20.8%), NNT 4.8, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 7.
|
|
risk of mechanical ventilation, 89.1% lower, RR 0.11, p = 0.05, treatment 0 of 25 (0.0%), control 4 of 24 (16.7%), NNT 6.0, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 7.
|
|
risk of ICU admission, 89.1% lower, RR 0.11, p = 0.05, treatment 0 of 25 (0.0%), control 4 of 24 (16.7%), NNT 6.0, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 7.
|
|
risk of no hospital discharge, 72.6% lower, RR 0.27, p = 0.07, treatment 2 of 25 (8.0%), control 7 of 24 (29.2%), NNT 4.7, day 14.
|
|
risk of no hospital discharge, 58.9% lower, RR 0.41, p = 0.02, treatment 6 of 25 (24.0%), control 14 of 24 (58.3%), NNT 2.9, day 7.
|
|
hospitalization time, 37.5% lower, relative time 0.62, p = 0.008, treatment median 5.0 IQR 4.0 n=25, control median 8.0 IQR 6.0 n=24.
|
|
relative WHO score, 50.0% better, RR 0.50, p = 0.04, treatment 22, control 24, day 7.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Gérain et al., 22 Jun 2023, Randomized Controlled Trial, Belgium, peer-reviewed, 8 authors, study period 1 April, 2021 - 29 October, 2021, this trial uses multiple treatments in the treatment arm (combined with quercetin) - results of individual treatments may vary, trial NCT04844658 (history).
Contact: melanie.uebelhoer@artialis.com.
NASAFYTOL® supplementation in adults hospitalized with COVID-19 infection: results from an exploratory open-label randomized controlled trial
Frontiers in Nutrition, doi:10.3389/fnut.2023.1137407
Objectives: The effect and safety of Nasafytol ® , a food supplement combining curcumin, quercetin, and Vitamin D, on hospitalized COVID-19-positive patients as support to standard of care were to be assessed.
Methods: This exploratory, open-label, randomized, controlled trial was carried out among hospitalized adults with COVID-19 infection. Participants were randomly assigned to receive Nasafytol ® or Fultium ® control. The improvement of the clinical condition and occurrence of (serious) adverse events were evaluated. The study was registered on clincaltrials.gov with the identifier NCT04844658. Results: Twenty-five patients received Nasafytol ® , and 24 received Fultium ® . Demographic characteristics were well balanced between the groups. On day 14 (or at hospital leave if < 14 days), no difference was observed between groups regarding their clinical condition, fever, or the need of oxygen therapy. At day 7, however, 19 participants had been discharged from the hospital in the Nasafytol ® arm compared to 10 participants in the Fultium ® arm. No participants were transferred to the ICU or died in the Nasafytol ® arm, vs. 4 transfers and 1 death in the Fultium ® arm. The clinical condition of participants in the Nasafytol ® arm had improved, as evidenced by a decrease in the COVID-19 WHO score. Interestingly, five SAEs occurred with Fultium ® , while no SAE was observed with Nasafytol ® . Conclusion: Supplementation with Nasafytol ® , in addition to standard-of-care treatment, led to a faster discharge from the hospital, improved clinical conditions of participants, and a reduced risk of serious outcomes, including transfer to the intensive care unit or death, in patients hospitalized with COVID-19.
Ethics statement The studies involving human participants were reviewed and approved by Ethics Committee of the University Hospital of Brussels (Erasme-ULB) in Belgium (Comité d'Ethique Hospitalo-Facultaire Erasme-ULB) (protocol code B4062020000305 on January 15, 2021). The patients/participants provided their written informed consent to participate in this study.
Author contributions BC and YH: conceptualization. JG, BC, JH, and YH: methodology. A-FD and JM: statistical analysis. JG: investigation. MU and YH: writing-original draft preparation. JG, BC, JH, SP, A-FD, and JM: writing-review and editing. MU, BC, and JH: project administration. JG, MU, BC, JH, SP, A-FD, JM, and YH had full access to all the data in the study and had final responsibility for the decision to submit for publication. BC, JH, and YH accessed and verified the data. All authors contributed to the article and approved the submitted version.
Conflict of interest YH is the founder and president of Artialis SA, a spin-off company of the University of Liège. YH has also received consulting and speaker fees from Tilman SA, Nestlé, Laboratoire Expanscience, Heel,
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed..
References
Aburto, Cisterna, Acuña, Ruíz, Viscardi et al., Obesity as a risk factor for severe COVID-19 in hospitalized patients: epidemiology and potential mechanisms, Healthcare, doi:10.3390/healthcare10101838
Ader, Bouscambert-Duchamp, Hites, Peiffer-Smadja, Poissy et al., Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial, Lancet Infect Dis, doi:10.1016/S1473-3099(21)00485-0
Andres, Pevny, Ziegenhagen, Bakhiya, Schäfer et al., Safety aspects of the use of quercetin as a dietary supplement, Mol Nutr Food Res, doi:10.1002/mnfr.201700447
Babaei, Nassiri-Asl, Hosseinzadeh, Curcumin (a constituent of turmeric): new treatment option against COVID-19, Food Sci Nutr, doi:10.1002/fsn3.1858
Bayati, Noroozi, Ghanbari-Jahromi, Jalali, Inequality in the distribution of Covid-19 vaccine: a systematic review, IJEqH, doi:10.1186/s12939-022-01729-x
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the treatment of Covid-19-final report, N Engl J Med, doi:10.1056/NEJMoa2007764
Bilezikian, Bikle, Hewison, Lazaretti-Castro, Formenti et al., MECHANISMS IN ENDOCRINOLOGY: vitamin D and COVID-19, Eur J Endocrinol, doi:10.1530/EJE-20-0665
Cheng, Hsu, Lin, Hsu, Ho et al., Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions, Anticancer Res
Choi, Song, Kwon, Quercetin 3-rhamnoside exerts antiinfluenza a virus activity in mice, Phytother Res, doi:10.1002/ptr.3529
Dai, Gu, Su, Wang, Zhao et al., Inhibition of curcumin on influenza a virus infection and influenza pneumonia via oxidative stress, TLR2/4, p38/ JNK MAPK and NF-κB pathways, Int Immunopharmacol, doi:10.1016/j.intimp.2017.11.009
Grant, Lahore, Mcdonnell, Baggerly, French et al., Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths, Nutrients, doi:10.3390/nu12040988
Han, Xu, Guo, Huang, Curcumin ameliorates severe influenza pneumonia via attenuating lung injury and regulating macrophage cytokines production, Clin Exp Pharmacol Physiol, doi:10.1111/1440-1681.12848
Khan, Iqtadar, Mumtaz, Heinrich, Pascual-Figal et al., Oral co-supplementation of curcumin, quercetin, and vitamin D3 as an adjuvant therapy for mild to moderate symptoms of COVID-19-results from a pilot open-label, randomized controlled trial, Front Pharmacol, doi:10.3389/fphar.2022.898062
Lee, Yu, Trimpert, Benthani, Mairhofer et al., Virusinduced senescence is a driver and therapeutic target in COVID-19, Nature, doi:10.1038/s41586-021-03995-1
Leyva-López, Gutierrez-Grijalva, Perez, Heredia, Flavonoids as cytokine modulators: a possible therapy for inflammation-related diseases, Int J Mol Sci, doi:10.3390/ijms17060921
Mercola, Grant, Wagner, Evidence regarding vitamin D and risk of COVID-19 and its severity, Nutrients, doi:10.3390/nu12113361
Moscatelli, Sessa, Valenzano, Polito, Monda et al., COVID-19: role of nutrition and supplementation, Nutrients, doi:10.3390/nu13030976
Murai, Fernandes, Sales, Pinto, Goessler et al., Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2020.26848
Park, Yoon, Kim, Lee, Chong, Synthesis and antiviral evaluation of 7-O-arylmethylquercetin derivatives against SARS-associated coronavirus (SCV) and hepatitis C virus (HCV), Arch Pharm Res, doi:10.1007/s12272-012-0108-9
Pierro, Derosa, Maffioli, Bertuccioli, Togni et al., Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: a prospective, randomized, controlled, and open-label study, Int J Gen Med, doi:10.2147/IJGM.S318720
Qiu, Kroeker, He, Kozak, Audet et al., Prophylactic efficacy of quercetin 3-β-O-d-glucoside against Ebola virus infection, Antimicrob Agents Chemother, doi:10.1128/AAC.00307-16
Recovery Collaborative Group, Horby, Lim, Emberson, Mafham et al., Dexamethasone in hospitalized patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Sabico, Enani, Sheshah, Aljohani, Aldisi et al., Effects of a 2-week 5000 IU versus 1000 IU vitamin D3 supplementation on recovery of symptoms in patients with mild to moderate Covid-19: a randomized clinical trial, Nutrients, doi:10.3390/nu13072170
Sharma, Prateeksha, Singh, Singh, Rao et al., Nanocurcumin potently inhibits SARS-CoV-2 spike protein-induced cytokine storm by deactivation of MAPK/NF-κB Signaling in epithelial cells, ACS Appl Bio Mater, doi:10.1021/acsabm.1c00874
Sterne, Murthy, Diaz, Slutsky, Villar, Association between Administration of Systemic Corticosteroids and Mortality among Critically ill Patients with COVID-19: a meta-analysis, JAMA, doi:10.1001/jama.2020.17023
Vahedian-Azimi, Abbasifard, Rahimi-Bashar, Guest, Majeed et al., Effectiveness of curcumin on outcomes of hospitalized COVID-19 patients: a systematic review of clinical trials, Nutrients, doi:10.3390/nu14020256
Who, Trial Consortiumpan, Peto, Henao-Restrepo, Preziosi et al., Repurposed antiviral drugs for Covid-19-interim WHO solidarity trial results, N Engl J Med, doi:10.1056/NEJMoa2023184
Wu, Effect of curcumin on nasal symptoms and airflow in patients with perennial allergic rhinitis, Ann Allergy Asthma Immunol, doi:10.1016/j.anai.2016.09.427
Wu, Li, Li, He, Jiang et al., Quercetin as an antiviral agent inhibits influenza a virus (IAV) entry. Viruses, doi:10.3390/v8010006
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet, doi:10.1016/S0140-6736(20)30566-3
DOI record:
{
"DOI": "10.3389/fnut.2023.1137407",
"ISSN": [
"2296-861X"
],
"URL": "http://dx.doi.org/10.3389/fnut.2023.1137407",
"abstract": "<jats:sec><jats:title>Objectives</jats:title><jats:p>The effect and safety of Nasafytol<jats:sup>®</jats:sup>, a food supplement combining curcumin, quercetin, and Vitamin D, on hospitalized COVID-19-positive patients as support to standard of care were to be assessed.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>This exploratory, open-label, randomized, controlled trial was carried out among hospitalized adults with COVID-19 infection. Participants were randomly assigned to receive Nasafytol<jats:sup>®</jats:sup> or Fultium<jats:sup>®</jats:sup> control. The improvement of the clinical condition and occurrence of (serious) adverse events were evaluated. The study was registered on <jats:ext-link>clincaltrials.gov</jats:ext-link> with the identifier NCT04844658.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Twenty-five patients received Nasafytol<jats:sup>®</jats:sup>, and 24 received Fultium<jats:sup>®</jats:sup>. Demographic characteristics were well balanced between the groups. On day 14 (or at hospital leave if &lt; 14 days), no difference was observed between groups regarding their clinical condition, fever, or the need of oxygen therapy. At day 7, however, 19 participants had been discharged from the hospital in the Nasafytol<jats:sup>®</jats:sup> arm compared to 10 participants in the Fultium<jats:sup>®</jats:sup> arm. No participants were transferred to the ICU or died in the Nasafytol<jats:sup>®</jats:sup> arm, vs. 4 transfers and 1 death in the Fultium<jats:sup>®</jats:sup> arm. The clinical condition of participants in the Nasafytol<jats:sup>®</jats:sup> arm had improved, as evidenced by a decrease in the COVID-19 WHO score. Interestingly, five SAEs occurred with Fultium<jats:sup>®</jats:sup>, while no SAE was observed with Nasafytol<jats:sup>®</jats:sup>.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Supplementation with Nasafytol<jats:sup>®</jats:sup>, in addition to standard-of-care treatment, led to a faster discharge from the hospital, improved clinical conditions of participants, and a reduced risk of serious outcomes, including transfer to the intensive care unit or death, in patients hospitalized with COVID-19.</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fnut.2023.1137407"
],
"author": [
{
"affiliation": [],
"family": "Gérain",
"given": "Jean",
"sequence": "first"
},
{
"affiliation": [],
"family": "Uebelhoer",
"given": "Melanie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Costes",
"given": "Bérénice",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Herman",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pietri",
"given": "Sandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Donneau",
"given": "Anne-Françoise",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Monseur",
"given": "Justine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Henrotin",
"given": "Yves",
"sequence": "additional"
}
],
"container-title": "Frontiers in Nutrition",
"container-title-short": "Front. Nutr.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2023,
6,
23
]
],
"date-time": "2023-06-23T16:46:23Z",
"timestamp": 1687538783000
},
"deposited": {
"date-parts": [
[
2023,
6,
23
]
],
"date-time": "2023-06-23T16:46:27Z",
"timestamp": 1687538787000
},
"indexed": {
"date-parts": [
[
2023,
6,
24
]
],
"date-time": "2023-06-24T04:19:05Z",
"timestamp": 1687580345138
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
6,
22
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
22
]
],
"date-time": "2023-06-22T00:00:00Z",
"timestamp": 1687392000000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fnut.2023.1137407/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2023,
6,
22
]
]
},
"published-online": {
"date-parts": [
[
2023,
6,
22
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "publisher",
"first-page": "1054",
"journal-title": "Lancet",
"key": "ref1",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1186/s12939-022-01729-x",
"article-title": "Inequality in the distribution of Covid-19 vaccine: a systematic review",
"author": "Bayati",
"doi-asserted-by": "publisher",
"first-page": "122",
"journal-title": "IJEqH",
"key": "ref2",
"volume": "21",
"year": "2022"
},
{
"article-title": "Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results",
"author": "Pan",
"first-page": "497",
"key": "ref3",
"volume-title": "N Engl J Med",
"year": "2021"
},
{
"DOI": "10.1016/S1473-3099(21)00485-0",
"article-title": "Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial",
"author": "Ader",
"doi-asserted-by": "publisher",
"first-page": "209",
"journal-title": "Lancet Infect Dis",
"key": "ref4",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19—final report",
"author": "Beigel",
"doi-asserted-by": "publisher",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "ref5",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.17023",
"article-title": "Association between Administration of Systemic Corticosteroids and Mortality among Critically ill Patients with COVID-19: a meta-analysis",
"author": "Sterne",
"doi-asserted-by": "publisher",
"first-page": "1330",
"journal-title": "JAMA",
"key": "ref6",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with Covid-19",
"author": "Horby",
"doi-asserted-by": "publisher",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "ref7",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"article-title": "Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"doi-asserted-by": "publisher",
"first-page": "1637",
"journal-title": "Lancet",
"key": "ref8",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.3390/nu13030976",
"article-title": "COVID-19: role of nutrition and supplementation",
"author": "Moscatelli",
"doi-asserted-by": "publisher",
"first-page": "976",
"journal-title": "Nutrients",
"key": "ref9",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.3390/nu12040988",
"article-title": "Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths",
"author": "Grant",
"doi-asserted-by": "publisher",
"first-page": "988",
"journal-title": "Nutrients",
"key": "ref10",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.26848",
"article-title": "Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial",
"author": "Murai",
"doi-asserted-by": "publisher",
"first-page": "1053",
"journal-title": "JAMA",
"key": "ref11",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1007/s12272-012-0108-9",
"article-title": "Synthesis and antiviral evaluation of 7-O-arylmethylquercetin derivatives against SARS-associated coronavirus (SCV) and hepatitis C virus (HCV)",
"author": "Park",
"doi-asserted-by": "publisher",
"first-page": "77",
"journal-title": "Arch Pharm Res",
"key": "ref12",
"volume": "35",
"year": "2012"
},
{
"DOI": "10.1128/AAC.00307-16",
"article-title": "Prophylactic efficacy of quercetin 3-β-O-d-glucoside against Ebola virus infection",
"author": "Qiu",
"doi-asserted-by": "publisher",
"first-page": "5182",
"journal-title": "Antimicrob Agents Chemother",
"key": "ref13",
"volume": "60",
"year": "2016"
},
{
"DOI": "10.3390/v8010006",
"article-title": "Quercetin as an antiviral agent inhibits influenza a virus (IAV) entry",
"author": "Wu",
"doi-asserted-by": "publisher",
"first-page": "6",
"journal-title": "Viruses",
"key": "ref14",
"volume": "8",
"year": "2015"
},
{
"DOI": "10.1111/1440-1681.12848",
"article-title": "Curcumin ameliorates severe influenza pneumonia via attenuating lung injury and regulating macrophage cytokines production",
"author": "Han",
"doi-asserted-by": "publisher",
"first-page": "84",
"journal-title": "Clin Exp Pharmacol Physiol",
"key": "ref15",
"volume": "45",
"year": "2018"
},
{
"DOI": "10.1016/j.intimp.2017.11.009",
"article-title": "Inhibition of curcumin on influenza a virus infection and influenza pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF-κB pathways",
"author": "Dai",
"doi-asserted-by": "publisher",
"first-page": "177",
"journal-title": "Int Immunopharmacol",
"key": "ref16",
"volume": "54",
"year": "2018"
},
{
"DOI": "10.1002/ptr.3529",
"article-title": "Quercetin 3-rhamnoside exerts antiinfluenza a virus activity in mice",
"author": "Choi",
"doi-asserted-by": "publisher",
"first-page": "462",
"journal-title": "Phytother Res",
"key": "ref17",
"volume": "26",
"year": "2012"
},
{
"DOI": "10.1002/fsn3.1858",
"article-title": "Curcumin (a constituent of turmeric): new treatment option against COVID-19",
"author": "Babaei",
"doi-asserted-by": "publisher",
"first-page": "5215",
"journal-title": "Food Sci Nutr",
"key": "ref18",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.anai.2016.09.427",
"article-title": "Effect of curcumin on nasal symptoms and airflow in patients with perennial allergic rhinitis",
"author": "Wu",
"doi-asserted-by": "publisher",
"first-page": "697",
"journal-title": "Ann Allergy Asthma Immunol",
"key": "ref19",
"volume": "117",
"year": "2016"
},
{
"article-title": "Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions",
"author": "Cheng",
"first-page": "2895",
"journal-title": "Anticancer Res",
"key": "ref20",
"volume": "21",
"year": "2001"
},
{
"DOI": "10.1002/mnfr.201700447",
"article-title": "Safety aspects of the use of quercetin as a dietary supplement",
"author": "Andres",
"doi-asserted-by": "publisher",
"first-page": "1700447",
"journal-title": "Mol Nutr Food Res",
"key": "ref21",
"volume": "62",
"year": "2018"
},
{
"DOI": "10.3389/fphar.2022.898062",
"article-title": "Oral co-supplementation of curcumin, quercetin, and vitamin D3 as an adjuvant therapy for mild to moderate symptoms of COVID-19-results from a pilot open-label, randomized controlled trial",
"author": "Khan",
"doi-asserted-by": "publisher",
"first-page": "1781",
"journal-title": "Front Pharmacol",
"key": "ref22",
"volume": "13",
"year": "2022"
},
{
"key": "ref23",
"volume-title": "WHO R&D blueprint novel coronavirus—COVID-19 THerapeutic trial synopsis",
"year": "2020"
},
{
"DOI": "10.3390/nu12113361",
"article-title": "Evidence regarding vitamin D and risk of COVID-19 and its severity",
"author": "Mercola",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Nutrients",
"key": "ref24",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.3390/nu13072170",
"article-title": "Effects of a 2-week 5000 IU versus 1000 IU vitamin D3 supplementation on recovery of symptoms in patients with mild to moderate Covid-19: a randomized clinical trial",
"author": "Sabico",
"doi-asserted-by": "publisher",
"first-page": "2170",
"journal-title": "Nutrients",
"key": "ref25",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.3390/nu14020256",
"article-title": "Effectiveness of curcumin on outcomes of hospitalized COVID-19 patients: a systematic review of clinical trials",
"author": "Vahedian-Azimi",
"doi-asserted-by": "publisher",
"first-page": "256",
"journal-title": "Nutrients",
"key": "ref26",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.2147/IJGM.S318720",
"article-title": "Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: a prospective, randomized, controlled, and open-label study",
"author": "Di Pierro",
"doi-asserted-by": "publisher",
"first-page": "2359",
"journal-title": "Int J Gen Med",
"key": "ref27",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1530/EJE-20-0665",
"article-title": "MECHANISMS IN ENDOCRINOLOGY: vitamin D and COVID-19",
"author": "Bilezikian",
"doi-asserted-by": "publisher",
"first-page": "R133",
"journal-title": "Eur J Endocrinol",
"key": "ref28",
"volume": "183",
"year": "2020"
},
{
"DOI": "10.1021/acsabm.1c00874",
"article-title": "Nanocurcumin potently inhibits SARS-CoV-2 spike protein-induced cytokine storm by deactivation of MAPK/NF-κB Signaling in epithelial cells",
"author": "Sharma",
"doi-asserted-by": "publisher",
"first-page": "483",
"journal-title": "ACS Appl Bio Mater",
"key": "ref29",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.3390/ijms17060921",
"article-title": "Flavonoids as cytokine modulators: a possible therapy for inflammation-related diseases",
"author": "Leyva-López",
"doi-asserted-by": "publisher",
"first-page": "921",
"journal-title": "Int J Mol Sci",
"key": "ref30",
"volume": "17",
"year": "2016"
},
{
"DOI": "10.1038/s41586-021-03995-1",
"article-title": "Virus-induced senescence is a driver and therapeutic target in COVID-19",
"author": "Lee",
"doi-asserted-by": "publisher",
"first-page": "283",
"journal-title": "Nature",
"key": "ref31",
"volume": "599",
"year": "2021"
},
{
"DOI": "10.3390/healthcare10101838",
"article-title": "Obesity as a risk factor for severe COVID-19 in hospitalized patients: epidemiology and potential mechanisms",
"author": "Aburto",
"doi-asserted-by": "publisher",
"first-page": "1838",
"journal-title": "Healthcare",
"key": "ref32",
"volume": "10",
"year": "2022"
},
{
"author": "Ross",
"key": "ref33",
"volume-title": "Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin DDietary Reference Intakes for Calcium and Vitamin D",
"year": "2011"
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fnut.2023.1137407/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Nutrition and Dietetics",
"Endocrinology, Diabetes and Metabolism",
"Food Science"
],
"subtitle": [],
"title": "NASAFYTOL® supplementation in adults hospitalized with COVID-19 infection: results from an exploratory open-label randomized controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "10"
}
gerain
