COVIDMED – An early pandemic randomized clinical trial of losartan treatment for hospitalized COVID-19 patients
et al., Contemporary Clinical Trials Communications, doi:10.1016/j.conctc.2022.100968, COVIDMED, NCT04340557, Oct 2022
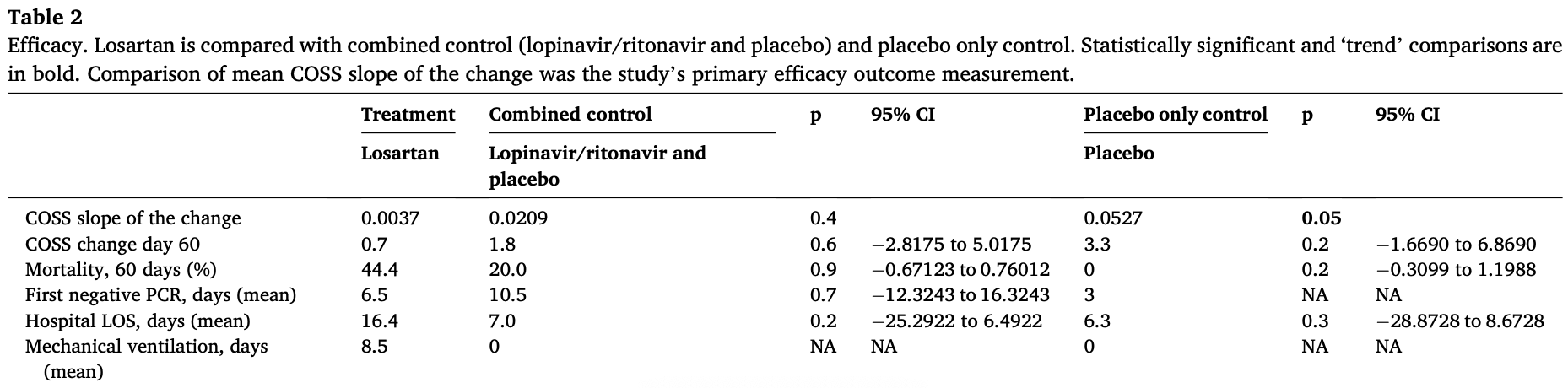
RCT 15 hospitalized COVID-19 patients showing no significant differences with losartan treatment. The study was terminated early due to low enrollment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 533.3% higher, RR 6.33, p = 0.49, treatment 4 of 9 (44.4%), control 0 of 3 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
hospitalization time, 160.3% higher, relative time 2.60, p = 0.30, treatment 9, control 3.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Freilich et al., 31 Oct 2022, Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, mean age 69.8, 4 authors, trial NCT04340557 (history) (COVIDMED).
Contact: daniel.freilich@bassett.org, jennifer.victory@bassett.org, paul.jenkins@bassett.org.
COVIDMED – An early pandemic randomized clinical trial of losartan treatment for hospitalized COVID-19 patients
Contemporary Clinical Trials Communications, doi:10.1016/j.conctc.2022.100968
Losartan Angiotensin converting enzyme inhibitor (ACEi) Angiotensin II receptor Blocker (ARB) COVID
Source of funding This work was supported by a Bassett Research Institute ED Thomas Grant (which included funds to purchase study drugs) and salary support from the Bassett Research Institute and Bassett Medical Center's Department of Internal Medicine. Funders had no role in the design, execution, data analysis, or manuscript preparation or publication of this study.
Authors' contributions All authors contributed to refinement of and approved the manuscript.
Authorship role All authors collaborated in the writing/editing of the manuscript.
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data Supplementary data to this article can be found online at https://doi. org/10.1016/j.conctc.2022.100968.
References
Baral, Tsampasian, Debski, Moran, Garg et al., Association between renin-angiotensin-aldosterone system inhibitors and clinical outcomes in patients with COVID-19A systematic review and meta-analysis, JAMA Netw. Open
Bean, Kraljevic, Searle, Bendayan, Kevin et al., Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID-19 infection in a multi-site UK acute hospital trust, Eur. J. Heart Fail
Bengtson, Montgomery, Nazir, Satterwhite, Kim et al., An open label trial to assess safety of losartan for treating worsening respiratory illness in COVID-19, Front. Med, doi:10.3389/fmed.2021.630209
Cao, Wang, Wen, Liu, Wang et al., A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19, N. Engl. J. Med
Cohen, Hanff, William, Sweitzer, Rosado-Santander et al., Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial, Lancet Respir. Med
Duarte, Pelorosso, Nicolosi, Salgado, Vetulli et al., Telmisartan for treatment of Covid-19 patients: an open multicenter randomized clinical trial, EClinicalMedicine, doi:10.1016/j.eclinm.2021.100962
Fosbol, Butt, Østergaard, Andersson, Selmer et al., Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality, JAMA
Geriak, Haddad, Kullar, Greenwood, Habib et al., Randomized prospective open label study shows No impact on clinical outcome of adding losartan to hospitalized COVID-19 patients with mild hypoxemia, Infect. Dis. Ther
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell
Horby, Mafham, Linsell, Bell, Staplin et al., Effect of hydroxychloroquine in hospitalized patients with covid-19. The RECOVERY collaborative group, N. Engl. J. Med
Ishiyama, Gallagher, Averill, Tallant, Brosnihan et al., Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors, Hypertension
Kuba, Imai, Rao, Gao, Guo et al., A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury, Nat. Med
Lopes, Macedo, De Barros E Silva, Moll-Bernardes, Santos et al., Effect of discontinuing vs continuing angiotensinconverting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19A randomized clinical trial, JAMA
Mancia, Rea, Ludergnani, Apolone, Corrao, Renin-Angiotensin-aldosterone system blockers and the risk of covid-19, N. Engl. J. Med
Mehra, Desai, Kuy, Henry, Patel, Cardiovascular disease, drug therapy, and mortality in covid-19, N. Engl. J. Med
Mehta, Kalra, Nowacki, Anjewierden, Han et al., Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19), JAMA Cardiol
Puskarich, Cummins, Ingraham, Wacker, Reilkoff et al., A multi-center phase II randomized clinical trial of losartan on symptomatic outpatients with COVID-19, EClinicalMedicine, doi:10.1016/j.eclinm.2021.100957
Puskarich, Ingraham, Merck, Efficacy of losartan in hospitalized patients with COVID-19-induced lung injury: a randomized clinical trial, JAMA Netw. Open
Reynolds, Adhikari, Pulgarin, Troxel, Iturrate et al., Renin-Angiotensin-aldosterone system inhibitors and risk of covid-19, N. Engl. J. Med
Vaduganathan, Vardeny, Michel, Mcmurray, Pfeffer et al., Renin-Angiotensin-aldosterone system inhibitors in patients with covid-19, N. Engl. J. Med
DOI record:
{
"DOI": "10.1016/j.conctc.2022.100968",
"ISSN": [
"2451-8654"
],
"URL": "http://dx.doi.org/10.1016/j.conctc.2022.100968",
"alternative-id": [
"S2451865422000850"
],
"article-number": "100968",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "COVIDMED – An early pandemic randomized clinical trial of losartan treatment for hospitalized COVID-19 patients"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Contemporary Clinical Trials Communications"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.conctc.2022.100968"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 The Authors. Published by Elsevier Inc."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-2219-6900",
"affiliation": [],
"authenticated-orcid": false,
"family": "Freilich",
"given": "Daniel",
"sequence": "first"
},
{
"affiliation": [],
"family": "Victory",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jenkins",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gadomski",
"given": "Anne",
"sequence": "additional"
}
],
"container-title": "Contemporary Clinical Trials Communications",
"container-title-short": "Contemporary Clinical Trials Communications",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
7,
20
]
],
"date-time": "2022-07-20T06:12:27Z",
"timestamp": 1658297547000
},
"deposited": {
"date-parts": [
[
2024,
2,
4
]
],
"date-time": "2024-02-04T14:01:11Z",
"timestamp": 1707055271000
},
"indexed": {
"date-parts": [
[
2024,
4,
24
]
],
"date-time": "2024-04-24T00:58:17Z",
"timestamp": 1713920297570
},
"is-referenced-by-count": 5,
"issued": {
"date-parts": [
[
2022,
10
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
1
]
],
"date-time": "2022-10-01T00:00:00Z",
"timestamp": 1664582400000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
14
]
],
"date-time": "2022-07-14T00:00:00Z",
"timestamp": 1657756800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2451865422000850?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2451865422000850?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "100968",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
10
]
]
},
"published-print": {
"date-parts": [
[
2022,
10
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "271",
"issue": "2",
"journal-title": "Cell",
"key": "10.1016/j.conctc.2022.100968_bib1",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1038/nm1267",
"article-title": "A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Letter",
"author": "Kuba",
"doi-asserted-by": "crossref",
"first-page": "875",
"journal-title": "Nat. Med.",
"key": "10.1016/j.conctc.2022.100968_bib2",
"volume": "11",
"year": "2005"
},
{
"DOI": "10.1161/01.HYP.0000124667.34652.1a",
"article-title": "Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors",
"author": "Ishiyama",
"doi-asserted-by": "crossref",
"first-page": "970",
"issue": "5",
"journal-title": "Hypertension",
"key": "10.1016/j.conctc.2022.100968_bib3",
"volume": "43",
"year": "2004"
},
{
"DOI": "10.1056/NEJMsr2005760",
"article-title": "Renin–Angiotensin–aldosterone system inhibitors in patients with covid-19",
"author": "Vaduganathan",
"doi-asserted-by": "crossref",
"first-page": "1653",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.conctc.2022.100968_bib4",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007621",
"article-title": "Cardiovascular disease, drug therapy, and mortality in covid-19",
"author": "Mehra",
"doi-asserted-by": "crossref",
"first-page": "e102",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.conctc.2022.100968_bib5",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2008975",
"article-title": "Renin–Angiotensin–aldosterone system inhibitors and risk of covid-19",
"author": "Reynolds",
"doi-asserted-by": "crossref",
"first-page": "2441",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.conctc.2022.100968_bib6",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2006923",
"article-title": "Renin–Angiotensin–aldosterone system blockers and the risk of covid-19",
"author": "Mancia",
"doi-asserted-by": "crossref",
"first-page": "2431",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.conctc.2022.100968_bib7",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1002/ejhf.1924",
"article-title": "Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID-19 infection in a multi-site UK acute hospital trust",
"author": "Bean",
"doi-asserted-by": "crossref",
"first-page": "967",
"issue": "6",
"journal-title": "Eur. J. Heart Fail.",
"key": "10.1016/j.conctc.2022.100968_bib8",
"volume": "22",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.11301",
"article-title": "Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality",
"author": "Fosbol",
"doi-asserted-by": "crossref",
"first-page": "168",
"issue": "2",
"journal-title": "JAMA",
"key": "10.1016/j.conctc.2022.100968_bib9",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1001/jamacardio.2020.1855",
"article-title": "Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19)",
"author": "Mehta",
"doi-asserted-by": "crossref",
"first-page": "1020",
"issue": "9",
"journal-title": "JAMA Cardiol.",
"key": "10.1016/j.conctc.2022.100968_bib10",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.25864",
"article-title": "Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19A randomized clinical trial",
"author": "Lopes",
"doi-asserted-by": "crossref",
"first-page": "254",
"issue": "3",
"journal-title": "JAMA",
"key": "10.1016/j.conctc.2022.100968_bib11",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(20)30558-0",
"article-title": "Continuation versus discontinuation of renin–angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial",
"author": "Cohen",
"doi-asserted-by": "crossref",
"first-page": "275",
"issue": "3",
"journal-title": "Lancet Respir. Med.",
"key": "10.1016/j.conctc.2022.100968_bib12",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.3389/fmed.2021.630209",
"article-title": "An open label trial to assess safety of losartan for treating worsening respiratory illness in COVID-19",
"author": "Bengtson",
"doi-asserted-by": "crossref",
"journal-title": "Front. Med. (Lausanne)",
"key": "10.1016/j.conctc.2022.100968_bib13",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2021.100962",
"article-title": "Telmisartan for treatment of Covid-19 patients: an open multicenter randomized clinical trial",
"author": "Duarte",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "10.1016/j.conctc.2022.100968_bib14",
"year": "2021"
},
{
"DOI": "10.1007/s40121-021-00453-3",
"article-title": "Randomized prospective open label study shows No impact on clinical outcome of adding losartan to hospitalized COVID-19 patients with mild hypoxemia",
"author": "Geriak",
"doi-asserted-by": "crossref",
"first-page": "1323",
"issue": "3",
"journal-title": "Infect. Dis. Ther.",
"key": "10.1016/j.conctc.2022.100968_bib15",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2021.3594",
"article-title": "Association between renin-angiotensin-aldosterone system inhibitors and clinical outcomes in patients with COVID-19A systematic review and meta-analysis",
"author": "Baral",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "JAMA Netw. Open",
"key": "10.1016/j.conctc.2022.100968_bib16",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2021.100957",
"article-title": "A multi-center phase II randomized clinical trial of losartan on symptomatic outpatients with COVID-19",
"author": "Puskarich",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "10.1016/j.conctc.2022.100968_bib17",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2022.2735",
"article-title": "Efficacy of losartan in hospitalized patients with COVID-19–induced lung injury: a randomized clinical trial",
"author": "Puskarich",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "JAMA Netw. Open",
"key": "10.1016/j.conctc.2022.100968_bib18",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2022926",
"article-title": "Effect of hydroxychloroquine in hospitalized patients with covid-19. The RECOVERY collaborative group",
"author": "Horby",
"doi-asserted-by": "crossref",
"first-page": "2030",
"issue": "21",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.conctc.2022.100968_bib19",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A trial of lopinavir–ritonavir in adults hospitalized with severe covid-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "1787",
"issue": "19",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.conctc.2022.100968_bib20",
"volume": "382",
"year": "2020"
}
],
"reference-count": 20,
"references-count": 20,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2022.01.12.22269095",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2451865422000850"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "COVIDMED – An early pandemic randomized clinical trial of losartan treatment for hospitalized COVID-19 patients",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "29"
}