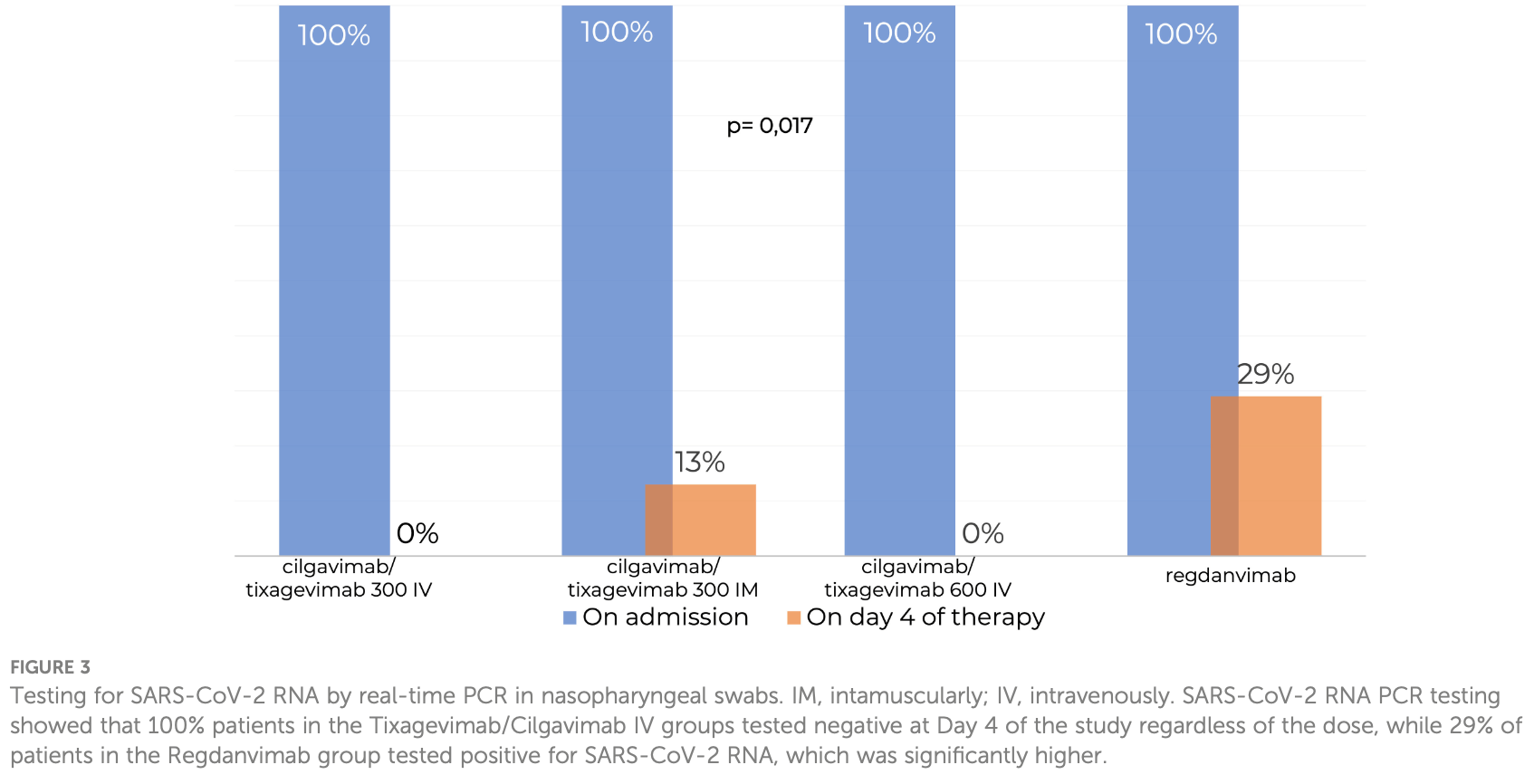
Real-world clinical effectiveness of Tixagevimab/Cilgavimab and Regdanvimab monoclonal antibodies for COVID-19 treatment in Omicron variant-dominant period
et al., Frontiers in Immunology, doi:10.3389/fimmu.2023.1259725, NCT05982704, Oct 2023
42nd treatment shown to reduce risk in
May 2022, now with p = 0.0066 from 19 studies, recognized in 33 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Prospective study of 77 COVID-19 outpatients showing improved efficacy with tixagevimab/cilgavimab compared to regdanvimab during Omicron variant dominance.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2.75.2, BA.4.6, BQ.1.11, BA.5, BA.2.75, XBB2,3, XBB.1.53, ХВВ.1.9.13, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.14.
Study covers tixagevimab/cilgavimab and regdanvimab.
1.
Planas et al., Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies, bioRxiv, doi:10.1101/2022.11.17.516888.
2.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Fomina et al., 20 Oct 2023, prospective, Russia, peer-reviewed, 16 authors, study period 20 August, 2022 - 1 February, 2023, this trial compares with another treatment - results may be better when compared to placebo, trial NCT05982704 (history).
Contact: marina.ivanova0808@yandex.ru.
Real-world clinical effectiveness of Tixagevimab/Cilgavimab and Regdanvimab monoclonal antibodies for COVID-19 treatment in Omicron variant-dominant period
Frontiers in Immunology, doi:10.3389/fimmu.2023.1259725
Several virus-neutralizing monoclonal antibodies (mAbs) have become new tools in the treatment of the coronavirus disease (COVID-19), but their effectiveness against the rapidly mutating virus is questionable. The present study investigated the effectiveness of Tixagevimab/Cilgavimab and Regdanvimab for mild and moderate COVID-19 treatment in real-world clinical practice during the Omicron variant-dominant period. Patients with known risk factors for disease progression and increasing disease severity were enrolled in the study within the first 7 days of symptom onset. Seventy-seven patients were divided into four groups: first 15 patients received 300 mg Tixagevimab/Cilgavimab intravenously (IV) and 23 patients got the same drug 300 mg intramuscularly (IM), the next 15 patients was on the same combination in dose of 600 mg IV, and 24 patients were on Regdanvimab at a dose of 40 mg/kg IV. By Day 4, 100% of Tixagevimab/ Cilgavimab IV patients showed negative polymerase chain reaction results for SARS-CoV-2 Ribonucleic acid (RNA) regardless of the mAbs dose while in the Regdanvimab group 29% of the patients were positive for SARS-CoV-2 virus RNA. The testing for virus neutralizing antibodies (nAbs) to various Omicron sublineages (BA.1, BA.2, and BA.5) showed that an increase in nAb levels was detected in blood serum immediately after the drug administration only in Tixagevimab/Cilgavimab 300 mg and 600 mg IV groups. In the group of Frontiers in Immunology frontiersin.org 01
Ethics statement The studies involving humans were approved by Local ethics committee City Clinical Hospital 52 (version 1.1 of 478 08.09.2022). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbasi, Researchers tie severe immunosuppression to chronic COVID-19 and virus variants, JAMA, doi:10.1001/jama.2021.7212
Akalin, Azzi, Bartash, Seethamraju, Parides et al., Covid-19 and kidney transplantation, N Engl J Med, doi:10.1056/NEJMc2011117
Aleem, Samad, Vaqar, Emerging variants of SARS-coV-2 and novel therapeutics against coronavirus (COVID-19)
Bender Ignacio, Wohl, Arends, Reddy, Mu et al., Comparative pharmacokinetics of tixagevimab/cilgavimab (AZD7442) administered intravenously versus intramuscularly in symptomatic SARS-coV-2 infection, Clin Pharmacol Ther, doi:10.1002/cpt.2706
Bigdelou, Sepand, Najafikhoshnoo, Negrete, Sharaf et al., COVID-19 and preexisting comorbidities: risks, synergies, and clinical outcomes, Front Immunol, doi:10.3389/fimmu.2022.890517
Boschi, Colson, Bancod, Moal, Scola, Omicron variant escapes therapeutic monoclonal antibodies (mAbs) including recently released evusheld ® , contrary to 8 prior main variant of concern (VOC), Clin Infect Dis, doi:10.1093/cid/ciac143
Bruel, Hadjadj, Maes, Planas, Seve et al., Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies, Nat Med, doi:10.1038/s41591-022-01792-5
Bruel, Stefc, Nguyen, Toniutti, Staropoli et al., Longitudinal analysis of serum neutralization of SARS-CoV-2 Omicron BA.2, BA.4, and BA.5 in patients receiving monoclonal antibodies, Cell Rep Med, doi:10.1016/j.xcrm.2022.100850
Cameroni, Bowen, Rosen, Saliba, Zepeda et al., Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift, Nature, doi:10.1038/s41586-021-04386-2
Cao, Yisimayi, Jian, Song, Wang, 2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection, Nature, doi:10.1038/s41586-022-04980-y
Crooke, Ovsyannikova, Poland, Kennedy, Immunosenescence and human vaccine immune responses, Immun Ageing, doi:10.1186/s12979-019-0164-9
Dougan, Nirula, Azizad, Mocherla, Gottlieb et al., BLAZE-1 investigators. Bamlanivimab plus etesevimab in mild or moderate covid-19, N Engl J Med, doi:10.1056/NEJMoa2102685
Favalli, Ingegnoli, Lucia, Cincinelli, Cimaz et al., COVID-19 infection and rheumatoid arthritis: Faraway, so close!, Autoimmun Rev, doi:10.1016/j.autrev.2020.102523
Fomina, Lebedkina, Markina, Kriulin, Kotenko et al., SARS-CoV-2-neutralising monoclonal antibodies: mechanism of action and research results, Pediatria N.A. G.N. Speransky, doi:10.24110/0031-403X-2022-101-3-156-169
Gruell, Vanshylla, Tober-Lau, Hillus, Schommers et al., mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant, Nat Med, doi:10.1038/s41591-021-01676-0
Gupta, Gonzalez-Rojas, Juarez, Casal, Moya et al., Early treatment for covid-19 with SARS-coV-2 neutralizing antibody sotrovimab, N Engl J Med, doi:10.1056/NEJMoa2107934
Hu, Hu, Chu, Yau, Zhang et al., In-silico analysis of monoclonal antibodies against SARS-coV-2 omicron, Viruses, doi:10.3390/v14020390
Jensen, Stromme, Moyassari, Chadha, Tartaglia et al., COVID-19 vaccines: Considering sex differences in efficacy and safety, Contemp Clin Trials, doi:10.1016/j.cct.2022.106700
Keam, Tixagevimab + Cilgavimab: first approval, Drugs, doi:10.1007/s40265-022-01731-1
Mares, Hartung, Multiple sclerosis and COVID-19, Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub, doi:10.5507/bp.2020.033
Mаrkina, Fomina, Lebedkina, Kruglova, Chernov et al., Efficacy and safety of regdanvimab in patients with mild/ moderate COVID-19 and high risk of progression of the disease: a retrospective study in a short-term stay unit, Terapevticheskii Arkhiv (Ter Arkh), doi:10.26442/00403660.2022.05.201690
Nadesalingam, Cantoni, Aguinam, Chan, Paloniemi et al., Vaccination and protective immunity to SARS-CoV-2 omicron variants in people with immunodeficiencies, Lancet Microbe, doi:10.1016/S2666-5247(22)00297-X
Nasreen, Chung, He, Brown, Gubbay et al., Effectiveness of COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe outcomes with variants of concern in Ontario, Nat Microbiol, doi:10.1038/s41564-021-01053-0
Notarte, Catahay, Peligro, Velasco, Ver et al., Humoral response in hemodialysis patients post-SARS-coV-2 mRNA vaccination: A systematic review of literature, Vaccines, doi:10.3390/vaccines11040724
O'brien, Forleo-Neto, Musser, Chan, Sarkar, Subcutaneous REGEN-COV antibody combination to prevent covid-19, N Engl J Med, doi:10.1056/NEJMoa2109682
O'horo, Challener, Speicher, Bosch, Seville et al., Effectiveness of monoclonal antibodies in preventing severe COVID-19 with emergence of the delta variant, Mayo Clin Proc, doi:10.1016/j.mayocp.2021.12.002
Ocon, Ocon, Battaglia, Low, Neupane et al., Real-world effectiveness of tixagevimab and cilgavimab (Evusheld) in patients with hematological Malignancies, J Hematol, doi:10.14740/jh1062
Pal, Bhadada, Misra, COVID-19 vaccination in patients with diabetes mellitus: Current concepts, uncertainties and challenges, Diabetes Metab Syndr, doi:10.1016/j.dsx.2021.02.026
Parker, Desai, Marti, Nohynek, Kaslow et al., Response to additional COVID-19 vaccine doses in people who are immunocompromised: a rapid review, Lancet Glob Health, doi:10.1016/S2214-109X(21)00593-3
Razonable, Pawlowski, Horo, Arndt, Arndt et al., Casirivimab-Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19, Lancet's Eclinical Med, doi:10.1016/j.eclinm.2021.101102
Riccardi, Falcone, Yahav, Vaccination for SARS-coV-2 in hematological patients, Acta Haematol, doi:10.1159/000523753
Roe, Brady, Schuko, Nguyen, Beloor et al., Molecular characterization of AZD7442 (Tixagevimab-cilgavimab) neutralization of SARS-coV-2 omicron sublineages, Microbiol Spectr, doi:10.1128/spectrum.00333-23
Sharma, Notarte, Fernandez, Lippi, Gromiha et al., In silico evaluation of the impact of Omicron variant of concern sublineage BA.4 and BA.5 on the sensitivity of RT-qPCR assays for SARS-CoV-2 detection using whole genome sequencing, J Med Virol, doi:10.1002/jmv.28241
Singson, Kirley, Pham, Rothrock, Armistead et al., Factors associated with severe outcomes among immunocompromised adults hospitalized for COVID-19 -COVID-NET, 10 states, march 2020-february 2022, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7127a3
Slomski, Evusheld reduces COVID-19 disease severity among unvaccinated adults, JAMA, doi:10.1001/jama.2022.12178
Spihlman, Gadi, Wu, Moulton, COVID-19 and systemic lupus erythematosus: focus on immune response and therapeutics, Front Immunol, doi:10.3389/fimmu.2020.589474
Streinu-Cercel, Sandulescu, Preotescu, Kim, Kim et al., Efficacy and safety of regdanvimab (CT-P59): A phase 2/3 randomized, double-blind, placebo-controlled trial in outpatients with mild-to-moderate coronavirus disease 2019, Open Forum Infect Dis, doi:10.1093/ofid/ofac053
Takashita, Kinoshita, Yamayoshi, Sakai-Tagawa, Fujisaki et al., Efficacy of antibodies and antiviral drugs against covid-19 omicron variant, N Engl J Med, doi:10.1056/NEJMc2119407
Tegally, Moir, Everatt, Giovanetti, Scheepers et al., Emergence of SARS-coV-2 omicron lineages BA.4 and BA.5 in South Africa, Nat Med, doi:10.1038/s41591-022-01911-2
Vanblargan, Errico, Halfmann, Zost, Crowe et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies, Nat Med, doi:10.1038/s41591-021-01678-y
Vijenthira, Gong, Fox, Booth, Cook et al., Outcomes of patients with hematologic Malignancies and COVID-19: a systematic review and metaanalysis of 3377 patients, Blood, doi:10.1182/blood.2020008824
Zuidema, Pieters, Duchateau, Release and absorption rate aspects of intramuscularly injected pharmaceuticals, Int J Pharm, doi:10.1016/0378-5173(94)90103-1
DOI record:
{
"DOI": "10.3389/fimmu.2023.1259725",
"ISSN": [
"1664-3224"
],
"URL": "http://dx.doi.org/10.3389/fimmu.2023.1259725",
"abstract": "<jats:p>Several virus-neutralizing monoclonal antibodies (mAbs) have become new tools in the treatment of the coronavirus disease (COVID-19), but their effectiveness against the rapidly mutating virus is questionable. The present study investigated the effectiveness of Tixagevimab/Cilgavimab and Regdanvimab for mild and moderate COVID-19 treatment in real-world clinical practice during the Omicron variant-dominant period. Patients with known risk factors for disease progression and increasing disease severity were enrolled in the study within the first 7 days of symptom onset. Seventy-seven patients were divided into four groups: first 15 patients received 300 mg Tixagevimab/Cilgavimab intravenously (IV) and 23 patients got the same drug 300 mg intramuscularly (IM), the next 15 patients was on the same combination in dose of 600 mg IV, and 24 patients were on Regdanvimab at a dose of 40 mg/kg IV. By Day 4, 100% of Tixagevimab/Cilgavimab IV patients showed negative polymerase chain reaction results for SARS-CoV-2 Ribonucleic acid (RNA) regardless of the mAbs dose while in the Regdanvimab group 29% of the patients were positive for SARS-CoV-2 virus RNA. The testing for virus neutralizing antibodies (nAbs) to various Omicron sublineages (BA.1, BA.2, and BA.5) showed that an increase in nAb levels was detected in blood serum immediately after the drug administration only in Tixagevimab/Cilgavimab 300 mg and 600 mg IV groups. In the group of intravenous Regdanvimab, a significant increase in the level of nAbs to the Wuhan variant was detected immediately after the drug administration, while no increase in nAbs to different Omicron sublineages was observed.</jats:p><jats:sec><jats:title>Clinical trial registration</jats:title><jats:p><jats:uri>https://clinicaltrials.gov/</jats:uri>, identifier NCT05982704.</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fimmu.2023.1259725"
],
"author": [
{
"affiliation": [],
"family": "Fomina",
"given": "Daria S.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Lebedkina",
"given": "Marina S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Iliukhina",
"given": "Anna A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kovyrshina",
"given": "Anna V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shelkov",
"given": "Artem Y.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Andreev",
"given": "Sergey S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chernov",
"given": "Anton A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dolzhikova",
"given": "Inna V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kruglova",
"given": "Tatyana S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Andrenova",
"given": "Gerelma V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tukhvatulin",
"given": "Amir I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shcheblyakov",
"given": "Dmitry V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Karaulov",
"given": "Alexander V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lysenko",
"given": "Maryana A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Logunov",
"given": "Denis Y.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gintsburg",
"given": "Alexander L.",
"sequence": "additional"
}
],
"container-title": "Frontiers in Immunology",
"container-title-short": "Front. Immunol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2023,
10,
20
]
],
"date-time": "2023-10-20T13:21:47Z",
"timestamp": 1697808107000
},
"deposited": {
"date-parts": [
[
2023,
10,
20
]
],
"date-time": "2023-10-20T13:21:50Z",
"timestamp": 1697808110000
},
"indexed": {
"date-parts": [
[
2025,
4,
2
]
],
"date-time": "2025-04-02T15:03:29Z",
"timestamp": 1743606209419
},
"is-referenced-by-count": 2,
"issued": {
"date-parts": [
[
2023,
10,
20
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
10,
20
]
],
"date-time": "2023-10-20T00:00:00Z",
"timestamp": 1697760000000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fimmu.2023.1259725/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2023,
10,
20
]
]
},
"published-online": {
"date-parts": [
[
2023,
10,
20
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1038/s41564-021-01053-0",
"article-title": "Effectiveness of COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe outcomes with variants of concern in Ontario",
"author": "Nasreen",
"doi-asserted-by": "publisher",
"journal-title": "Nat Microbiol",
"key": "B1",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1016/S2666-5247(22)00297-X",
"article-title": "Vaccination and protective immunity to SARS-CoV-2 omicron variants in people with immunodeficiencies",
"author": "Nadesalingam",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Microbe",
"key": "B2",
"volume": "4",
"year": "2023"
},
{
"DOI": "10.1016/S2214-109X(21)00593-3",
"article-title": "Response to additional COVID-19 vaccine doses in people who are immunocompromised: a rapid review",
"author": "Parker",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Glob Health",
"key": "B3",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1182/blood.2020008824",
"article-title": "Outcomes of patients with hematologic Malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients",
"author": "Vijenthira",
"doi-asserted-by": "publisher",
"journal-title": "Blood",
"key": "B4",
"volume": "136",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2020.589474",
"article-title": "COVID-19 and systemic lupus erythematosus: focus on immune response and therapeutics",
"author": "Spihlman",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B5",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.5507/bp.2020.033",
"article-title": "Multiple sclerosis and COVID-19",
"author": "Mares",
"doi-asserted-by": "publisher",
"journal-title": "Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub",
"key": "B6",
"volume": "164",
"year": "2020"
},
{
"DOI": "10.1016/j.autrev.2020.102523",
"article-title": "COVID-19 infection and rheumatoid arthritis: Faraway, so close",
"author": "Favalli",
"doi-asserted-by": "publisher",
"journal-title": "Autoimmun Rev",
"key": "B7",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2011117",
"article-title": "Covid-19 and kidney transplantation",
"author": "Akalin",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B8",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1186/s12979-019-0164-9",
"article-title": "Immunosenescence and human vaccine immune responses",
"author": "Crooke",
"doi-asserted-by": "publisher",
"journal-title": "Immun Ageing",
"key": "B9",
"volume": "16",
"year": "2019"
},
{
"DOI": "10.15585/mmwr.mm7127a3",
"article-title": "Factors associated with severe outcomes among immunocompromised adults hospitalized for COVID-19 - COVID-NET, 10 states, march 2020-february 2022",
"author": "Singson",
"doi-asserted-by": "publisher",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "B10",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1001/jama.2021.7212",
"article-title": "Researchers tie severe immunosuppression to chronic COVID-19 and virus variants",
"author": "Abbasi",
"doi-asserted-by": "publisher",
"journal-title": "JAMA",
"key": "B11",
"volume": "325",
"year": "2021"
},
{
"key": "B12",
"unstructured": "AstraZeneca PharmaceuticalsLP\n Immunocompromised populations and the risk of viral variants. AstraZeneca Pharmaceuticals LP website2022"
},
{
"DOI": "10.3389/fimmu.2022.890517",
"article-title": "COVID-19 and preexisting comorbidities: risks, synergies, and clinical outcomes",
"author": "Bigdelou",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B13",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.3390/vaccines11040724",
"article-title": "Humoral response in hemodialysis patients post-SARS-coV-2 mRNA vaccination: A systematic review of literature",
"author": "Notarte",
"doi-asserted-by": "publisher",
"journal-title": "Vaccines (Basel)",
"key": "B14",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1016/j.dsx.2021.02.026",
"article-title": "COVID-19 vaccination in patients with diabetes mellitus: Current concepts, uncertainties and challenges",
"author": "Pal",
"doi-asserted-by": "publisher",
"journal-title": "Diabetes Metab Syndr",
"key": "B15",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1159/000523753",
"article-title": "Vaccination for SARS-coV-2 in hematological patients",
"author": "Riccardi",
"doi-asserted-by": "publisher",
"journal-title": "Acta Haematol",
"key": "B16",
"volume": "145",
"year": "2022"
},
{
"DOI": "10.1016/j.cct.2022.106700",
"article-title": "COVID-19 vaccines: Considering sex differences in efficacy and safety",
"author": "Jensen",
"doi-asserted-by": "publisher",
"journal-title": "Contemp Clin Trials",
"key": "B17",
"volume": "115",
"year": "2022"
},
{
"DOI": "10.14740/jh1062",
"article-title": "Real-world effectiveness of tixagevimab and cilgavimab (Evusheld) in patients with hematological Malignancies",
"author": "Ocon",
"doi-asserted-by": "publisher",
"first-page": "210",
"journal-title": "J Hematol",
"key": "B18",
"volume": "11",
"year": "2022"
},
{
"key": "B19",
"unstructured": "FDA Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 20202020"
},
{
"key": "B20",
"unstructured": "FDA Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 20202020"
},
{
"key": "B21",
"unstructured": "Coronavirus (COVID-19) Update: FDA Authorizes New Long-Acting Monoclonal Antibodies for Pre-exposure Prevention of COVID-19 in Certain Individuals"
},
{
"DOI": "10.24110/0031-403X-2022-101-3-156-169",
"article-title": "SARS-CoV-2-neutralising monoclonal antibodies: mechanism of action and research results",
"author": "Fomina",
"doi-asserted-by": "publisher",
"journal-title": "Pediatria N.A. G.N. Speransky",
"key": "B22",
"volume": "101",
"year": "2022"
},
{
"DOI": "10.1001/jama.2022.12178",
"article-title": "Evusheld reduces COVID-19 disease severity among unvaccinated adults",
"author": "Slomski",
"doi-asserted-by": "publisher",
"first-page": "322",
"journal-title": "JAMA",
"key": "B23",
"volume": "328",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2119407",
"article-title": "Efficacy of antibodies and antiviral drugs against covid-19 omicron variant",
"author": "Takashita",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B24",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/j.mayocp.2021.12.002",
"article-title": "Effectiveness of monoclonal antibodies in preventing severe COVID-19 with emergence of the delta variant",
"author": "O’Horo",
"doi-asserted-by": "publisher",
"journal-title": "Mayo Clin Proc",
"key": "B25",
"volume": "97",
"year": "2022"
},
{
"DOI": "10.1038/s41591-021-01676-0",
"article-title": "mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant",
"author": "Gruell",
"doi-asserted-by": "publisher",
"journal-title": "Nat Med",
"key": "B26",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1002/jmv.28241",
"article-title": "In silico evaluation of the impact of Omicron variant of concern sublineage BA.4 and BA.5 on the sensitivity of RT-qPCR assays for SARS-CoV-2 detection using whole genome sequencing",
"author": "Sharma",
"doi-asserted-by": "publisher",
"first-page": "e28241",
"journal-title": "J Med Virol",
"key": "B27",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "Early treatment for covid-19 with SARS-coV-2 neutralizing antibody sotrovimab",
"author": "Gupta",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B28",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1093/ofid/ofac053",
"article-title": "Efficacy and safety of regdanvimab (CT-P59): A phase 2/3 randomized, double-blind, placebo-controlled trial in outpatients with mild-to-moderate coronavirus disease 2019",
"author": "Streinu-Cercel",
"doi-asserted-by": "publisher",
"journal-title": "Open Forum Infect Dis",
"key": "B29",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1016/j.eclinm.2021.101102",
"article-title": "Casirivimab– Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19",
"author": "Razonable",
"doi-asserted-by": "publisher",
"journal-title": "Lancet’s Eclinical Med",
"key": "B30",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2109682",
"article-title": "Subcutaneous REGEN-COV antibody combination to prevent covid-19",
"author": "O’Brien",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B31",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2102685",
"article-title": "BLAZE-1 investigators. Bamlanivimab plus etesevimab in mild or moderate covid-19",
"author": "Dougan",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B32",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(22)00215-6",
"article-title": "ACTIV-3–Therapeutics for Inpatients with COVID-19 (TICO) Study Group. Tixagevimab-cilgavimab for treatment of patients hospitalised with COVID-19: a randomised, double-blind, phase 3 trial",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Respir Med",
"key": "B33",
"volume": "10",
"year": "2022"
},
{
"article-title": "Emerging variants of SARS-coV-2 and novel therapeutics against coronavirus (COVID-19)",
"author": "Aleem",
"key": "B34",
"volume-title": "StatPearls",
"year": "2023"
},
{
"DOI": "10.1007/s40265-022-01731-1",
"article-title": "Tixagevimab + Cilgavimab: first approval",
"author": "Keam",
"doi-asserted-by": "publisher",
"journal-title": "Drugs",
"key": "B35",
"volume": "82",
"year": "2022"
},
{
"key": "B36",
"unstructured": "Prevention, diagnosis, and treatment of novel coronavirus infection (COVID-19). Version 16 (08/18/2022)"
},
{
"DOI": "10.1038/s41591-022-01911-2",
"article-title": "Emergence of SARS-coV-2 omicron lineages BA.4 and BA.5 in South Africa",
"author": "Tegally",
"doi-asserted-by": "publisher",
"journal-title": "Nat Med",
"key": "B37",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1038/s41591-021-01678-y",
"article-title": "An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies",
"author": "VanBlargan",
"doi-asserted-by": "publisher",
"journal-title": "Nat Med",
"key": "B38",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-04386-2",
"article-title": "Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift",
"author": "Cameroni",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B39",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.1016/j.xcrm.2022.100850",
"article-title": "Longitudinal analysis of serum neutralization of SARS-CoV-2 Omicron BA.2, BA.4, and BA.5 in patients receiving monoclonal antibodies",
"author": "Bruel",
"doi-asserted-by": "publisher",
"journal-title": "Cell Rep Med",
"key": "B40",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.26442/00403660.2022.05.201690",
"article-title": "Efficacy and safety of regdanvimab in patients with mild/moderate COVID-19 and high risk of progression of the disease: a retrospective study in a short-term stay unit",
"author": "Mаrkina",
"doi-asserted-by": "publisher",
"journal-title": "Terapevticheskii Arkhiv (Ter Arkh)",
"key": "B41",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04980-y",
"article-title": "BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection",
"author": "Cao",
"doi-asserted-by": "publisher",
"first-page": "593",
"journal-title": "Nature",
"key": "B42",
"volume": "608",
"year": "2022"
},
{
"DOI": "10.3390/v14020390",
"article-title": "In-silico analysis of monoclonal antibodies against SARS-coV-2 omicron",
"author": "Hu",
"doi-asserted-by": "publisher",
"journal-title": "Viruses",
"key": "B43",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac143",
"article-title": "Omicron variant escapes therapeutic monoclonal antibodies (mAbs) including recently released evusheld®, contrary to 8 prior main variant of concern (VOC)",
"author": "Boschi",
"doi-asserted-by": "publisher",
"journal-title": "Clin Infect Dis",
"key": "B44",
"volume": "75",
"year": "2022"
},
{
"DOI": "10.1128/spectrum.00333-23",
"article-title": "Molecular characterization of AZD7442 (Tixagevimab-cilgavimab) neutralization of SARS-coV-2 omicron sublineages",
"author": "Roe",
"doi-asserted-by": "publisher",
"first-page": "e0033323",
"journal-title": "Microbiol Spectr",
"key": "B45",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1038/s41591-022-01792-5",
"article-title": "Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies",
"author": "Bruel",
"doi-asserted-by": "publisher",
"journal-title": "Nat Med",
"key": "B46",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1002/cpt.2706",
"article-title": "Comparative pharmacokinetics of tixagevimab/cilgavimab (AZD7442) administered intravenously versus intramuscularly in symptomatic SARS-coV-2 infection",
"author": "Bender Ignacio",
"doi-asserted-by": "publisher",
"journal-title": "Clin Pharmacol Ther",
"key": "B47",
"volume": "112",
"year": "2022"
},
{
"DOI": "10.1016/0378-5173(94)90103-1",
"article-title": "Release and absorption rate aspects of intramuscularly injected pharmaceuticals",
"author": "Zuidema",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Int J Pharm",
"key": "B48",
"volume": "47",
"year": "1988"
}
],
"reference-count": 48,
"references-count": 48,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fimmu.2023.1259725/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Real-world clinical effectiveness of Tixagevimab/Cilgavimab and Regdanvimab monoclonal antibodies for COVID-19 treatment in Omicron variant-dominant period",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "14"
}
