Evaluation of adalimumab effects in managing severe cases of COVID-19: A randomized controlled trial
et al., International Immunopharmacology, doi:10.1016/j.intimp.2021.107961, IRCT20151227025726N23, Oct 2021
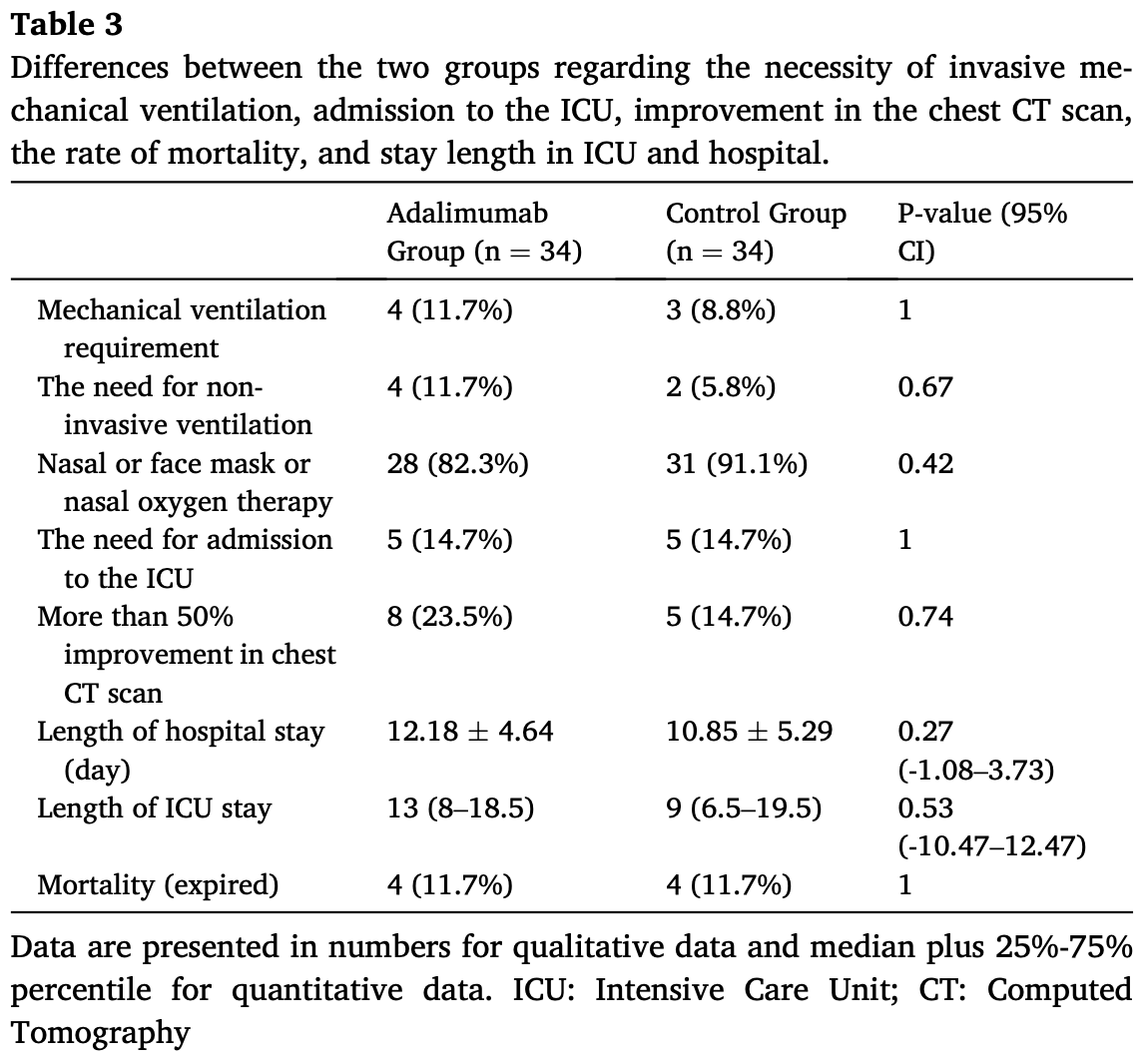
RCT 68 hospitalized patients with severe COVID-19 showing no significant differences with adalimumab treatment.
|
risk of death, no change, RR 1.00, p = 1.00, treatment 4 of 34 (11.8%), control 4 of 34 (11.8%).
|
|
risk of mechanical ventilation, 33.3% higher, RR 1.33, p = 1.00, treatment 4 of 34 (11.8%), control 3 of 34 (8.8%).
|
|
risk of ICU admission, no change, RR 1.00, p = 1.00, treatment 5 of 34 (14.7%), control 5 of 34 (14.7%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Fakharian et al., 31 Oct 2021, Randomized Controlled Trial, Iran, peer-reviewed, 10 authors, trial IRCT20151227025726N23.
Contact: fzh.dastan@gmail.com.
Evaluation of adalimumab effects in managing severe cases of COVID-19: A randomized controlled trial
International Immunopharmacology, doi:10.1016/j.intimp.2021.107961
Background: COVID-19, which is a disease caused by the SARS-CoV-2 virus, has spread around the world since late 2019. Studies have found associations between the rising levels of TNF-α and severe COVID-19 cases. Hence, TNF-α blocking can possibly be a favorable intervention in modifying COVID-19. To this end, in order to manage pneumonia caused by COVID-19, adalimumab may potentially be considered as a potential therapeutic agent. The present study aimed to investigate the potential therapeutic role of adalimumab in treating COVID-19 cases in combination therapy with remdesivir and dexamethasone. Methods: Among the 68 patients who were included in the current randomized controlled trial, 34 were assigned to the adalimumab group and the remaining 34 were assigned to the control group. Adalimumab at a dose of 40 mg, subcutaneous for once, was used for the intervention group. Both the intervention and control groups received remdesivir, dexamethasone, and supportive care. The data gathered to make comparisons of the groups included demographic information, the rate of mortality, mechanical ventilation requirement, length of stay in hospital and Intensive Care Unit (ICU), and imaging findings. Results: There was no significant difference between the two groups in the terms of mortality rate (P-value = 1) and mechanical ventilation requirement (P-value = 1). The length of hospital and ICU stay as well as radiologic changes were not affected either (P-value = 1, 0.27, and 0.53, respectively). Conclusions: Our findings did not support the use of adalimumab in combination with remdesivir and dexamethasone in the treatment of severe COVID-19 cases.
References
Baden, Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine, N Engl J Med
Bai, Updated guidance on the management of COVID-19: from an American Thoracic Society/European Respiratory Society coordinated International Task Force (29 July 2020), Eur Respir Rev
Brito, COVID-19 in patients with rheumatological diseases treated with anti-TNF, Annals of the rheumatic diseases
Chen, Clinical and immunological features of severe and moderate coronavirus disease 2019, The Journal of clinical investigation
Chen, Yan, Man, TNFα inhibitor may be effective for severe COVID-19: learning from toxic epidermal necrolysis, Ther Adv Respir Dis
Collange, Coronavirus Disease 2019: Associated Multiple Organ Damage, Open Forum Infect Dis
Conti, Evolution of COVID-19 infection in 4 psoriatic patients treated with biological drugs, Journal of the European Academy of Dermatology and Venereology
Dastan, Promising effects of tocilizumab in COVID-19: A non-controlled, prospective clinical trial, International immunopharmacology
Del, Valle, An inflammatory cytokine signature predicts COVID-19 severity and survival, Nat Med
Fakharian, Successful Management of COVID-19 With Adalimumab in a Post-Coronary Artery Bypass Graft Surgery Patient, J Cardiothorac Vasc Anesth
Feldmann, Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed, The Lancet
Guan, Clinical Characteristics of Coronavirus Disease 2019 in China, New England Journal of Medicine
Lai, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges, International Journal of Antimicrobial Agents
Nguyen, Immunoglobulins in the treatment of COVID-19 infection: Proceed with caution!, Clinical Immunology
Ozkan, How does colistin-induced nephropathy develop and can it be treated?, Antimicrobial agents and chemotherapy
Solun, Shoenfeld, Inhibition of metalloproteinases in therapy for severe lung injury due to COVID-19, Med Drug Discov
Tufan, Guler, Matucci-Cerinic, COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs, Turk J Med Sci
Tursi, COVID-19 infection in Crohn's disease under treatment with adalimumab, Gut
Tursi, Vetrone, Papa, Anti-TNF-α Agents in Inflammatory Bowel Disease and Course of COVID-19, Inflammatory Bowel Diseases
Vabret, None, Immunology of COVID-19: Current State of the Science, Immunity
Verity, Estimates of the severity of coronavirus disease 2019: a modelbased analysis, Lancet Infect Dis
Wang, Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China, JAMA
Wu, An Update on Current Therapeutic Drugs Treating COVID-19, Current pharmacology reports
Zhou, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet
DOI record:
{
"DOI": "10.1016/j.intimp.2021.107961",
"ISSN": [
"1567-5769"
],
"URL": "http://dx.doi.org/10.1016/j.intimp.2021.107961",
"alternative-id": [
"S156757692100597X"
],
"article-number": "107961",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Evaluation of adalimumab effects in managing severe cases of COVID-19: A randomized controlled trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "International Immunopharmacology"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.intimp.2021.107961"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 Elsevier B.V. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Fakharian",
"given": "Atefeh",
"sequence": "first"
},
{
"affiliation": [],
"family": "Barati",
"given": "Saghar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mirenayat",
"given": "Maryam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rezaei",
"given": "Mitra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haseli",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Torkaman",
"given": "Pooria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yousefian",
"given": "Sahar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dastan",
"given": "Alireza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "jamaati",
"given": "Hamidreza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dastan",
"given": "Farzaneh",
"sequence": "additional"
}
],
"container-title": "International Immunopharmacology",
"container-title-short": "International Immunopharmacology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
7,
7
]
],
"date-time": "2021-07-07T07:43:52Z",
"timestamp": 1625643832000
},
"deposited": {
"date-parts": [
[
2024,
11,
5
]
],
"date-time": "2024-11-05T20:13:37Z",
"timestamp": 1730837617000
},
"funder": [
{
"DOI": "10.13039/501100005851",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100005851",
"id-type": "DOI"
}
],
"name": "Shahid Beheshti University of Medical Sciences"
}
],
"indexed": {
"date-parts": [
[
2025,
2,
21
]
],
"date-time": "2025-02-21T04:40:29Z",
"timestamp": 1740112829003,
"version": "3.37.3"
},
"is-referenced-by-count": 25,
"issued": {
"date-parts": [
[
2021,
10
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
1
]
],
"date-time": "2021-10-01T00:00:00Z",
"timestamp": 1633046400000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
1
]
],
"date-time": "2021-10-01T00:00:00Z",
"timestamp": 1633046400000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
1
]
],
"date-time": "2021-10-01T00:00:00Z",
"timestamp": 1633046400000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
1
]
],
"date-time": "2021-10-01T00:00:00Z",
"timestamp": 1633046400000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
1
]
],
"date-time": "2021-10-01T00:00:00Z",
"timestamp": 1633046400000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
1
]
],
"date-time": "2021-10-01T00:00:00Z",
"timestamp": 1633046400000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
1
]
],
"date-time": "2021-10-01T00:00:00Z",
"timestamp": 1633046400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S156757692100597X?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S156757692100597X?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "107961",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
10
]
]
},
"published-print": {
"date-parts": [
[
2021,
10
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/j.immuni.2020.05.002",
"article-title": "Immunology of COVID-19: Current State of the Science",
"author": "Vabret",
"doi-asserted-by": "crossref",
"first-page": "910",
"issue": "6",
"journal-title": "Immunity",
"key": "10.1016/j.intimp.2021.107961_b0005",
"volume": "52",
"year": "2020"
},
{
"DOI": "10.1016/j.clim.2020.108459",
"article-title": "Immunoglobulins in the treatment of COVID-19 infection: Proceed with caution!",
"author": "Nguyen",
"doi-asserted-by": "crossref",
"journal-title": "Clinical Immunology",
"key": "10.1016/j.intimp.2021.107961_b0010",
"volume": "216",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30243-7",
"article-title": "Estimates of the severity of coronavirus disease 2019: a model-based analysis",
"author": "Verity",
"doi-asserted-by": "crossref",
"first-page": "669",
"issue": "6",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.intimp.2021.107961_b0015",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.1585",
"article-title": "Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1061",
"issue": "11",
"journal-title": "China. JAMA",
"key": "10.1016/j.intimp.2021.107961_b0020",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"article-title": "Clinical Characteristics of Coronavirus Disease 2019 in China",
"author": "Guan",
"doi-asserted-by": "crossref",
"first-page": "1708",
"issue": "18",
"journal-title": "New England Journal of Medicine",
"key": "10.1016/j.intimp.2021.107961_b0025",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105924",
"article-title": "Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges",
"author": "Lai",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "International Journal of Antimicrobial Agents",
"key": "10.1016/j.intimp.2021.107961_b0030",
"volume": "55",
"year": "2020"
},
{
"article-title": "An Update on Current Therapeutic Drugs Treating COVID-19",
"author": "Wu",
"first-page": "1",
"journal-title": "Current pharmacology reports",
"key": "10.1016/j.intimp.2021.107961_b0035",
"year": "2020"
},
{
"DOI": "10.1128/AAC.00343-13",
"article-title": "How does colistin-induced nephropathy develop and can it be treated?",
"author": "Ozkan",
"doi-asserted-by": "crossref",
"first-page": "3463",
"issue": "8",
"journal-title": "Antimicrobial agents and chemotherapy",
"key": "10.1016/j.intimp.2021.107961_b0040",
"volume": "57",
"year": "2013"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"issue": "10229",
"journal-title": "Lancet",
"key": "10.1016/j.intimp.2021.107961_b0045",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.intimp.2020.106869",
"doi-asserted-by": "crossref",
"key": "10.1016/j.intimp.2021.107961_b0050",
"unstructured": "Dastan, F., et al., Promising effects of tocilizumab in COVID-19: A non-controlled, prospective clinical trial. International immunopharmacology, 2020. 88: p. 106869-106869."
},
{
"DOI": "10.1172/JCI137244",
"article-title": "Clinical and immunological features of severe and moderate coronavirus disease 2019",
"author": "Chen",
"doi-asserted-by": "crossref",
"issue": "5",
"journal-title": "The Journal of clinical investigation",
"key": "10.1016/j.intimp.2021.107961_b0055",
"volume": "130",
"year": "2020"
},
{
"article-title": "Successful Management of COVID-19 With Adalimumab in a Post-Coronary Artery Bypass Graft Surgery Patient",
"author": "Fakharian",
"journal-title": "J Cardiothorac Vasc Anesth",
"key": "10.1016/j.intimp.2021.107961_b0060",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(20)30858-8",
"article-title": "Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed",
"author": "Feldmann",
"doi-asserted-by": "crossref",
"first-page": "1407",
"issue": "10234",
"journal-title": "The Lancet",
"key": "10.1016/j.intimp.2021.107961_b0065",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1093/ofid/ofaa249",
"article-title": "Coronavirus Disease 2019: Associated Multiple Organ Damage",
"author": "Collange",
"doi-asserted-by": "crossref",
"first-page": "p. ofaa249",
"issue": "7",
"journal-title": "Open Forum Infect Dis",
"key": "10.1016/j.intimp.2021.107961_b0070",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1016/j.medidd.2020.100052",
"article-title": "Inhibition of metalloproteinases in therapy for severe lung injury due to COVID-19",
"author": "Solun",
"doi-asserted-by": "crossref",
"journal-title": "Med Drug Discov",
"key": "10.1016/j.intimp.2021.107961_b0075",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2035389",
"article-title": "Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine",
"author": "Baden",
"doi-asserted-by": "crossref",
"first-page": "403",
"issue": "5",
"journal-title": "N Engl J Med",
"key": "10.1016/j.intimp.2021.107961_b0080",
"volume": "384",
"year": "2021"
},
{
"article-title": "COVID-19 in patients with rheumatological diseases treated with anti-TNF",
"author": "Brito",
"journal-title": "Annals of the rheumatic diseases",
"key": "10.1016/j.intimp.2021.107961_b0085",
"year": "2020"
},
{
"DOI": "10.1136/gutjnl-2020-321240",
"article-title": "COVID-19 infection in Crohn’s disease under treatment with adalimumab",
"author": "Tursi",
"doi-asserted-by": "crossref",
"first-page": "1364",
"issue": "7",
"journal-title": "Gut",
"key": "10.1016/j.intimp.2021.107961_b0090",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.1111/jdv.16587",
"article-title": "Evolution of COVID-19 infection in 4 psoriatic patients treated with biological drugs",
"author": "Conti",
"doi-asserted-by": "crossref",
"journal-title": "Journal of the European Academy of Dermatology and Venereology",
"key": "10.1016/j.intimp.2021.107961_b0095",
"year": "2020"
},
{
"DOI": "10.3906/sag-2004-168",
"article-title": "COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs",
"author": "Tufan",
"doi-asserted-by": "crossref",
"first-page": "620",
"issue": "SI-1",
"journal-title": "Turk J Med Sci",
"key": "10.1016/j.intimp.2021.107961_b0100",
"volume": "50",
"year": "2020"
},
{
"DOI": "10.1093/ibd/izaa114",
"doi-asserted-by": "crossref",
"key": "10.1016/j.intimp.2021.107961_b0105",
"unstructured": "Tursi, A., L.M. Vetrone, and A. Papa, Anti-TNF-α Agents in Inflammatory Bowel Disease and Course of COVID-19. Inflammatory Bowel Diseases, 2020. 26(7): p. e73-e73."
},
{
"DOI": "10.1177/1753466620926800",
"doi-asserted-by": "crossref",
"key": "10.1016/j.intimp.2021.107961_b0110",
"unstructured": "Chen, X.Y., B.X. Yan, and X.Y. Man, TNFα inhibitor may be effective for severe COVID-19: learning from toxic epidermal necrolysis. Ther Adv Respir Dis, 2020. 14: p. 1753466620926800."
},
{
"DOI": "10.1038/s41591-020-1051-9",
"article-title": "An inflammatory cytokine signature predicts COVID-19 severity and survival",
"author": "Del Valle",
"doi-asserted-by": "crossref",
"first-page": "1636",
"issue": "10",
"journal-title": "Nat Med",
"key": "10.1016/j.intimp.2021.107961_b0115",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1183/16000617.0287-2020",
"article-title": "Updated guidance on the management of COVID-19: from an American Thoracic Society/European Respiratory Society coordinated International Task Force (29 July 2020)",
"author": "Bai",
"doi-asserted-by": "crossref",
"issue": "157",
"journal-title": "Eur Respir Rev",
"key": "10.1016/j.intimp.2021.107961_b0120",
"volume": "29",
"year": "2020"
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S156757692100597X"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"special_numbering": "C",
"subject": [],
"subtitle": [],
"title": "Evaluation of adalimumab effects in managing severe cases of COVID-19: A randomized controlled trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy",
"volume": "99"
}