Dec 18 2021 |
et al., Immunopathologia Persa, doi:10.34172/ipp.2022.30322 | A randomized clinical trial study on the efficacy and safety of adalimumab and methylprednisolone pulse therapy in the treatment of COVID-19 patients with acute respiratory distress syndrome |
| RCT 80 ICU patients with moderate to severe COVID-19 showing reduced hospital stay with adalimumab plus methylprednisolone compared to methylprednisolone alone, however authors excluded patients that died without reporting how many died. .. | ||
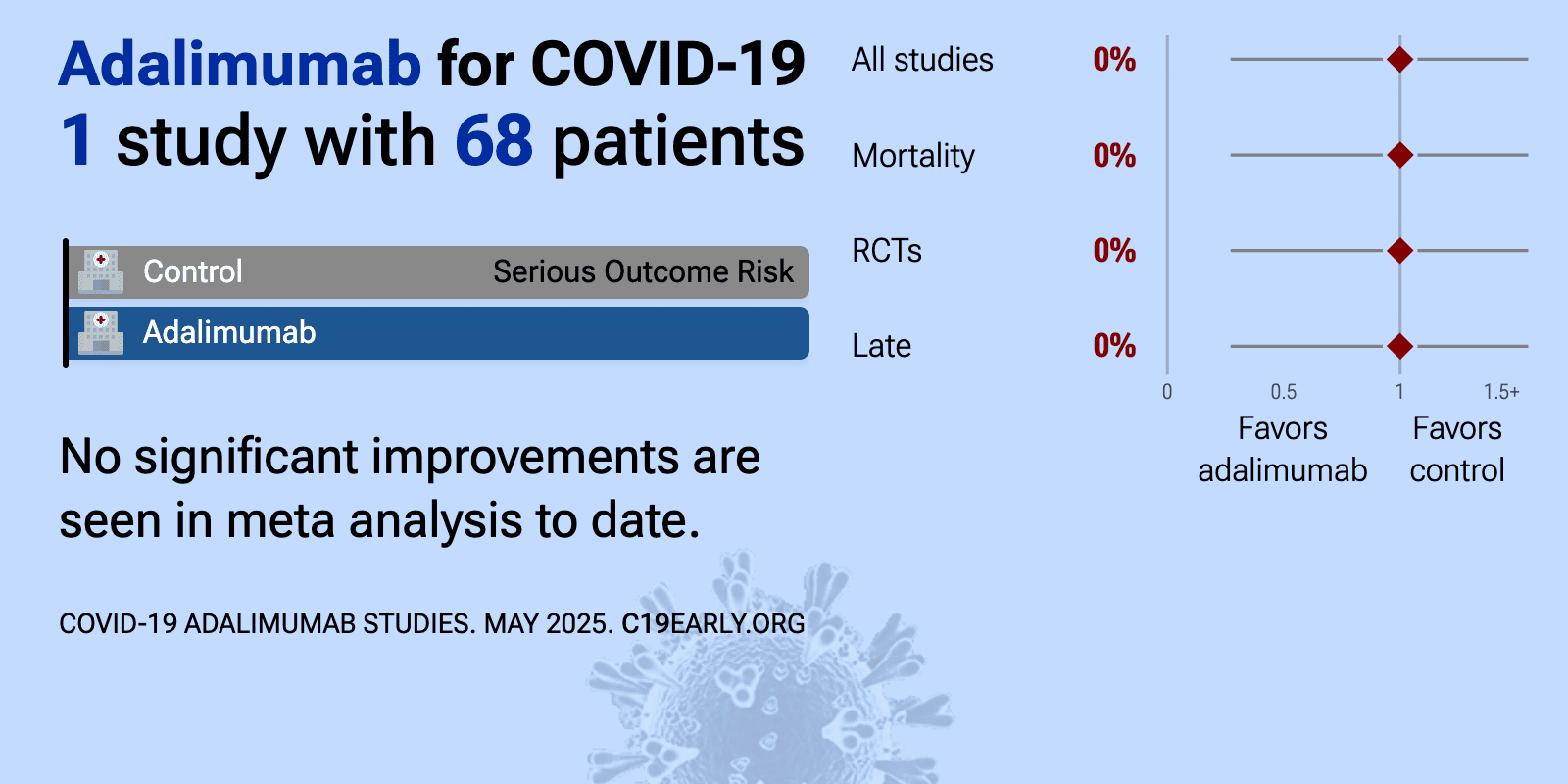
Oct 31 2021 |
et al., International Immunopharmacology, doi:10.1016/j.intimp.2021.107961 | Evaluation of adalimumab effects in managing severe cases of COVID-19: A randomized controlled trial |
| no change in mortality (p=1), 33% higher ventilation (p=1), and no change in ICU admission (p=1). RCT 68 hospitalized patients with severe COVID-19 showing no significant differences with adalimumab treatment. | ||