Association of Vitamin D Prescribing and Clinical Outcomes in Adults Hospitalized with COVID-19
et al., Nutrients, doi:10.3390/nu14153073, Jul 2022
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 135 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
N3C retrospective showing higher risk with vitamin D treatment for hospitalized patients. As noted by authors, confounding by indication may be significant. The more extreme ventilation result, which is a significant outlier among all studies, is consistent with such confounding. Timing, dose, and duration of treatment were not used.
Cholecalciferol was used in this study.
Meta-analysis shows that late stage treatment with calcitriol / calcifediol (or
paricalcitol, alfacalcidol, etc.) is more effective than cholecalciferol: 66% [47‑78%] lower risk vs. 45% [34‑54%] lower risk.
Cholecalciferol requires two hydroxylation steps to become activated - first
in the liver to calcifediol, then in the kidney to calcitriol. Calcitriol,
paricalcitol, and alfacalcidol are active vitamin D analogs that do not
require conversion. This allows them to have more rapid onset of action
compared to cholecalciferol. The time delay for cholecalciferol to increase
serum calcifediol levels can be 2-3 days, and the delay for converting
calcifediol to active calcitriol can be up to 7 days.
This is the 94th of 135 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
40 studies are RCTs, which show efficacy with p=0.0000001.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This study is excluded in the after exclusion results of meta-analysis:
substantial unadjusted confounding by indication likely.
|
risk of death, 8.9% higher, RR 1.09, p < 0.001, treatment 3,653 of 28,993 (12.6%), control 13,185 of 129,842 (10.2%), odds ratio converted to relative risk.
|
|
risk of mechanical ventilation, 40.8% higher, RR 1.41, p < 0.001, treatment 4,897 of 28,993 (16.9%), control 15,520 of 129,842 (12.0%), odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Fairfield et al., 26 Jul 2022, retrospective, USA, peer-reviewed, 10 authors, study period 1 January, 2020 - 31 July, 2021, dosage not specified.
Contact: kathleen.fairfield@mainehealth.org (corresponding author), kimberly.murray@mainehealth.org, susan.santangelo@mainehealth.org, clifford.rosen@mainehealth.org, alfred.anzalone@unmc.edu, william-beasley@ouhsc.edu, makhodaverdi@hsc.wvu.edu, slhodder@hsc.wvu.edu, owlhealthworks@gmail.com.
Association of Vitamin D Prescribing and Clinical Outcomes in Adults Hospitalized with COVID-19
Nutrients, doi:10.3390/nu14153073
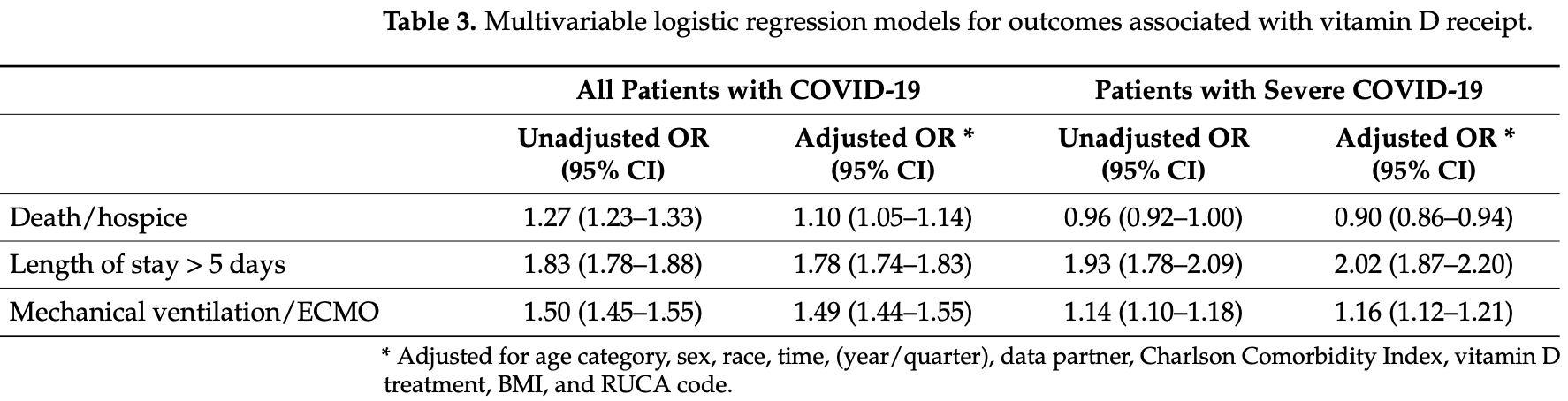
BackgroundIt is unclear whether vitamin D benefits inpatients with COVID-19. Objective: To examine the relationship between vitamin D and COVID-19 outcomes. Design: Cohort study. Setting: National COVID Cohort Collaborative (N3C) database. Patients: 158,835 patients with confirmed COVID-19 and a sub-cohort with severe disease (n = 81,381) hospitalized between 1 January 2020 and 31 July 2021. Methods: We identified vitamin D prescribing using codes for vitamin D and its derivatives. We created a sub-cohort defined as having severe disease as those who required mechanical ventilation or extracorporeal membrane oxygenation (ECMO), had hospitalization >5 days, or hospitalization ending in death or hospice. Using logistic regression, we adjusted for age, sex, race, BMI, Charlson Comorbidity Index, and urban/rural residence, time period, and study site. Outcomes of interest were death or transfer to hospice, longer length of stay, and mechanical ventilation/ECMO. Results: Patients treated with vitamin D were older, had more comorbidities, and higher BMI compared with patients who did not receive vitamin D. Vitamin D treatment was associated with an increased odds of death or referral for hospice (adjusted odds ratio (AOR) 1.10: 95% CI 1.05-1.14), hospital stay >5 days (AOR 1.78: 95% CI 1.74-1.83), and increased odds of mechanical ventilation/ECMO (AOR 1.49: 95% CI 1.44-1.55). In the sub-cohort of severe COVID-19, vitamin D decreased the odds of death or hospice (AOR 0.90, 95% CI 0.86-0.94), but increased the odds of hospital stay longer >5 days (AOR 2.03, 95% CI 1.87-2.21) and mechanical ventilation/ECMO (AOR 1.16, 95% CI 1.12-1.21). Limitations: Our findings could reflect more aggressive treatment due to higher severity. Conclusion: Vitamin D treatment was associated with greater odds of extended hospitalization, mechanical ventilation/ECMO, and death or hospice referral.
Author Contributions: Conceptualization, K.M.F., C.J.R., S.L.H. and K.A.M.; methodology, K.M.F., K.A.M., W.B. and C.J.R.; validation, J.H., W.B., A.J.A. and N3C Consortium; formal analysis, K.A.M., M.K., A.J.A. and K.M.F.; investigation, C.J.R. and S.L.H.; resources, S.S.; writing-original draft preparation, K.M.F. and K.A.M.; writing-review and editing, C.J.R. and S.S.; project administration, K.A.M.; funding acquisition, C.J.R., S.L.H. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding: The project described was supported by the National Institute of General Medical Sciences, 5U54GM104942-04. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Authorship was determined using ICMJE recommendations.
Institutional Review Board Statement: The N3C data transfer to NCATS is performed under a Johns Hopkins University Reliance Protocol # IRB00249128 or individual site agreements with NIH. The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board above. Informed Consent Statement: Patient consent was waived due to the fact this is a retrospective observational study using data from electronic medical records, and obtaining consent was not possible. Data Availability Statement: All diagnostic, medication, procedure, and laboratory concepts used in this study are available in Supplementary Table S7 . Raw code..
References
Adams, Hewison, Unexpected actions of vitamin D: New perspectives on the regulation of innate and adaptive immunity, Nat. Clin. Pract. Endocrinol. Metab, doi:10.1038/ncpendmet0716
Amrein, Schnedl, Holl, Riedl, Christopher et al., Effect of High-Dose Vitamin D 3 on Hospital Length of Stay in Critically Ill Patients with Vitamin D Deficiency: The VITdAL-ICU randomized clinical trial, JAMA, doi:10.1001/jama.2014.13204
Bennett, Moffitt, Hajagos, Amor, Anand et al., Clinical Characterization and Prediction of Clinical Severity of SARS-CoV-2 Infection Among US Adults Using Data From the US National COVID Cohort Collaborative, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2021.16901
Bhalla, Amento, Clemens, Holick, Krane, Specific high-affinity receptors for 1,25-dihydroxyvitamin D 3 in human peripheral blood mononuclear cells: Presence in monocytes and induction in t lymphocytes following activation, J. Clin. Endocrinol. Metab, doi:10.1210/jcem-57-6-1308
Bilezikian, Bikle, Hewison, Lazaretti-Castro, Formenti et al., Mechanisms in Endocrinology: Vitamin D and COVID-19, Eur. J. Endocrinol, doi:10.1530/EJE-20-0665
Bishop, Ismailova, Dimeloe, Hewison, White, Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory, JBMR Plus, doi:10.1002/jbm4.10405
Dancer, Parekh, Lax, D'souza, Zheng et al., Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS), Thorax, doi:10.1136/thoraxjnl-2014-206680
Deyo, Cherkin, Ciol, Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases, J. Clin. Epidemiol, doi:10.1016/0895-4356(92)90133-8
Fan, Johnson, Johnston, Elangovanraaj, Kothari et al., Monthly trend in mortality and length of stay among coronavirus disease 2019 (COVID-19) patients: Analysis of a nationwide multihospital US database, Infect. Control Hosp. Epidemiol, doi:10.1017/ice.2021.110
Fletcher, Bishop, Harrison, Swift, Cooper et al., Autoimmune disease and interconnections with vitamin D, Endocr. Connect, doi:10.1530/EC-21-0554
Greiller, Martineau, Modulation of the Immune Response to Respiratory Viruses by Vitamin, D. Nutrients, doi:10.3390/nu7064240
Gönen, Alaylıo Glu, Durcan, Özdemir, Şahin et al., Rapid and Effective Vitamin D Supplementation May Present Better Clinical Outcomes in COVID-19 (SARS-CoV-2) Patients by Altering Serum INOS1, IL1B, IFNg, Cathelicidin-LL37, and ICAM1, Nutrients, doi:10.3390/nu13114047
Haendel, Chute, Bennett, Eichmann, Guinney et al., The National COVID Cohort Collaborative (N3C): Rationale, design, infrastructure, and deployment, J. Am. Med. Inform. Assoc, doi:10.1093/jamia/ocaa196
Hastie, Pell, Sattar, Vitamin D and COVID-19 infection and mortality in UK Biobank, Eur. J. Nutr
Hewison, Vitamin D and the intracrinology of innate immunity, Mol. Cell. Endocrinol, doi:10.1016/j.mce.2010.02.013
Ho, Imai, King, Stuart, MatchIt: Nonparametric Preprocessing for Parametric Causal Inference, J. Stat. Softw, doi:10.18637/jss.v042.i08
Hosseini, El Abd, Ducharme, Effects of Vitamin D Supplementation on COVID-19 Related Outcomes: A Systematic Review and Meta-Analysis, Nutrients, doi:10.3390/nu14102134
Langlois, Szwec, D'aragon, Heyland, Manzanares, Vitamin D supplementation in the critically ill: A systematic review and meta-analysis, Clin. Nutr, doi:10.1016/j.clnu.2017.05.006
Looker, Dawson-Hughes, Calvo, Gunter, Sahyoun, Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III, Bone, doi:10.1016/S8756-3282(02)00692-0
Murai, Fernandes, Sales, Pinto, Goessler et al., Effect of a Single High Dose of Vitamin D 3 on Hospital Length of Stay in Patients with Moderate to Severe COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2020.26848
Nguyen, Chinn, Nahmias, Yuen, Kirby et al., Outcomes and Mortality Among Adults Hospitalized with COVID-19 at US Medical Centers, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2021.0417
Oristrell, Oliva, Casado, Subirana, Domínguez et al., Vitamin D supplementation and COVID-19 risk: A population-based, cohort study, J. Endocrinol. Investig, doi:10.1007/s40618-021-01639-9
Provvedini, Tsoukas, Deftos, Manolagas, 1 alpha,25-Dihydroxyvitamin D3-binding macromolecules in human B lymphocytes: Effects on immunoglobulin production, J. Immunol
Provvedini, Tsoukas, Deftos, Manolagas, 1,25-Dihydroxyvitamin D 3 Receptors in Human Leukocytes, Science, doi:10.1126/science.6310748
Reis, Fernandes, Sales, Santos, Dos Santos et al., Influence of vitamin D status on hospital length of stay and prognosis in hospitalized patients with moderate to severe COVID-19: A multicenter prospective cohort study, Am. J. Clin. Nutr, doi:10.1093/ajcn/nqab151
Sabico, Enani, Sheshah, Aljohani, Aldisi et al., Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate COVID-19: A Randomized Clinical Trial, Nutrients, doi:10.3390/nu13072170
Schleicher, Sternberg, Lacher, Sempos, Looker et al., The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin D shows recent modest increases, Am. J. Clin. Nutr, doi:10.3945/ajcn.115.127985
Shah, Varna, Sharma, Mavalankar, Does vitamin D supplementation reduce COVID-19 severity?-A systematic review, QJM, doi:10.1093/qjmed/hcac040
Tomaszewska, Rustecka, Lipi Ńska-Opałka, Piprek, Kloc et al., The Role of Vitamin D in COVID-19 and the Impact of Pandemic Restrictions on Vitamin D Blood Content, Front. Pharmacol, doi:10.3389/fphar.2022.836738
Weir, Thenappan, Bhargava, Chen, Does vitamin D deficiency increase the severity of COVID-19?, Clin. Med, doi:10.7861/clinmed.2020-0301
White, Emerging Roles of Vitamin D-Induced Antimicrobial Peptides in Antiviral Innate Immunity, Nutrients, doi:10.3390/nu14020284
Wortsman, Matsuoka, Chen, Lu, Holick, Decreased bioavailability of vitamin D in obesity, Am. J. Clin. Nutr, doi:10.1093/ajcn/72.3.690
Zajic, Amrein, Vitamin D deficiency in the ICU: A systematic review, Minerva Endocrinol
Zhang, Leung, Richers, Liu, Remigio et al., Vitamin D Inhibits Monocyte/Macrophage Proinflammatory Cytokine Production by Targeting MAPK Phosphatase-1, J. Immunol, doi:10.4049/jimmunol.1102412
DOI record:
{
"DOI": "10.3390/nu14153073",
"ISSN": [
"2072-6643"
],
"URL": "http://dx.doi.org/10.3390/nu14153073",
"abstract": "<jats:p>It is unclear whether vitamin D benefits inpatients with COVID-19. Objective: To examine the relationship between vitamin D and COVID-19 outcomes. Design: Cohort study. Setting: National COVID Cohort Collaborative (N3C) database. Patients: 158,835 patients with confirmed COVID-19 and a sub-cohort with severe disease (n = 81,381) hospitalized between 1 January 2020 and 31 July 2021. Methods: We identified vitamin D prescribing using codes for vitamin D and its derivatives. We created a sub-cohort defined as having severe disease as those who required mechanical ventilation or extracorporeal membrane oxygenation (ECMO), had hospitalization >5 days, or hospitalization ending in death or hospice. Using logistic regression, we adjusted for age, sex, race, BMI, Charlson Comorbidity Index, and urban/rural residence, time period, and study site. Outcomes of interest were death or transfer to hospice, longer length of stay, and mechanical ventilation/ECMO. Results: Patients treated with vitamin D were older, had more comorbidities, and higher BMI compared with patients who did not receive vitamin D. Vitamin D treatment was associated with an increased odds of death or referral for hospice (adjusted odds ratio (AOR) 1.10: 95% CI 1.05–1.14), hospital stay >5 days (AOR 1.78: 95% CI 1.74–1.83), and increased odds of mechanical ventilation/ECMO (AOR 1.49: 95% CI 1.44–1.55). In the sub-cohort of severe COVID-19, vitamin D decreased the odds of death or hospice (AOR 0.90, 95% CI 0.86–0.94), but increased the odds of hospital stay longer >5 days (AOR 2.03, 95% CI 1.87–2.21) and mechanical ventilation/ECMO (AOR 1.16, 95% CI 1.12–1.21). Limitations: Our findings could reflect more aggressive treatment due to higher severity. Conclusion: Vitamin D treatment was associated with greater odds of extended hospitalization, mechanical ventilation/ECMO, and death or hospice referral.</jats:p>",
"alternative-id": [
"nu14153073"
],
"author": [
{
"affiliation": [],
"family": "Fairfield",
"given": "Kathleen M.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Murray",
"given": "Kimberly A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3212-7845",
"affiliation": [],
"authenticated-orcid": false,
"family": "Anzalone",
"given": "A. Jerrod",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beasley",
"given": "William",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7559-1656",
"affiliation": [],
"authenticated-orcid": false,
"family": "Khodaverdi",
"given": "Maryam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hodder",
"given": "Sally L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harper",
"given": "Jeremy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santangelo",
"given": "Susan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rosen",
"given": "Clifford J.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "on behalf of the N3C Consortium",
"sequence": "additional"
}
],
"container-title": "Nutrients",
"container-title-short": "Nutrients",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
7,
27
]
],
"date-time": "2022-07-27T08:59:16Z",
"timestamp": 1658912356000
},
"deposited": {
"date-parts": [
[
2022,
7,
28
]
],
"date-time": "2022-07-28T08:41:28Z",
"timestamp": 1658997688000
},
"funder": [
{
"DOI": "10.13039/100006108",
"award": [
"U24 TR002306"
],
"doi-asserted-by": "publisher",
"name": "National Center for Advancing Translational Sciences"
}
],
"indexed": {
"date-parts": [
[
2022,
7,
28
]
],
"date-time": "2022-07-28T14:40:31Z",
"timestamp": 1659019231977
},
"is-referenced-by-count": 0,
"issue": "15",
"issued": {
"date-parts": [
[
2022,
7,
26
]
]
},
"journal-issue": {
"issue": "15",
"published-online": {
"date-parts": [
[
2022,
8
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
26
]
],
"date-time": "2022-07-26T00:00:00Z",
"timestamp": 1658793600000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2072-6643/14/15/3073/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "3073",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
7,
26
]
]
},
"published-online": {
"date-parts": [
[
2022,
7,
26
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"key": "ref1",
"unstructured": "WHO Coronavirus (COVID-19) Dashboard\nhttps://covid19.who.int"
},
{
"DOI": "10.1038/ncpendmet0716",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.1016/j.mce.2010.02.013",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1530/EC-21-0554",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1002/jbm4.10405",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1126/science.6310748",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"article-title": "1 alpha,25-Dihydroxyvitamin D3-binding macromolecules in human B lymphocytes: Effects on immunoglobulin production",
"author": "Provvedini",
"first-page": "2734",
"journal-title": "J. Immunol.",
"key": "ref7",
"volume": "136",
"year": "1986"
},
{
"DOI": "10.1210/jcem-57-6-1308",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.3390/nu7064240",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.3390/nu14020284",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.4049/jimmunol.1102412",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.1530/EJE-20-0665",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.3390/nu13114047",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1136/thoraxjnl-2014-206680",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1016/S8756-3282(02)00692-0",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1093/ajcn/72.3.690",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.3945/ajcn.115.127985",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1016/j.clnu.2017.05.006",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.3390/nu13072170",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1007/s00394-020-02372-4",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.3390/nu14102134",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.1001/jama.2020.26848",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.1093/jamia/ocaa196",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1001/jamanetworkopen.2021.16901",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1017/ice.2021.110",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1001/jamanetworkopen.2021.0417",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1016/0895-4356(92)90133-8",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.18637/jss.v042.i08",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.1093/ajcn/nqab151",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.1007/s40618-021-01639-9",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.1093/qjmed/hcac040",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.3389/fphar.2022.836738",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.7861/clinmed.2020-0301",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"article-title": "Vitamin D deficiency in the ICU: A systematic review",
"author": "Zajic",
"first-page": "275",
"journal-title": "Minerva Endocrinol.",
"key": "ref34",
"volume": "39",
"year": "2014"
},
{
"DOI": "10.1001/jama.2014.13204",
"doi-asserted-by": "publisher",
"key": "ref35"
}
],
"reference-count": 35,
"references-count": 35,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2072-6643/14/15/3073"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Food Science",
"Nutrition and Dietetics"
],
"subtitle": [],
"title": "Association of Vitamin D Prescribing and Clinical Outcomes in Adults Hospitalized with COVID-19",
"type": "journal-article",
"volume": "14"
}
