Assessment of toxicological effects of favipiravir (T‐705) on the lung tissue of rats: An experimental study
et al., Journal of Biochemical and Molecular Toxicology, doi:10.1002/jbt.23536, Nov 2023
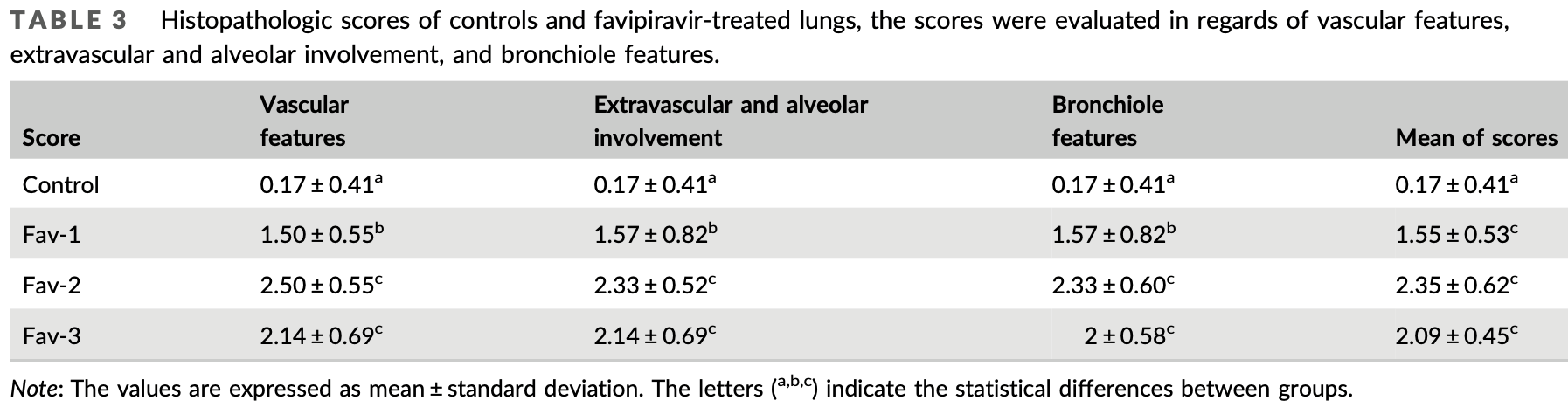
Analysis of the toxicological effects of favipiravir on healthy lung tissue in rats. Authors found that favipiravir treatment increased oxidative stress, apoptosis, and inflammation in the lung tissue as evidenced by changes in antioxidant parameters, increased expression of pro-apoptotic markers like Bax and caspase-3, and higher levels of inflammatory markers like NF-κB, IL-6, and P2X7R. The histopathological analysis also showed unfavorable changes like thickening in the alveolar regions, hemorrhage, and infiltration of inflammatory cells after favipiravir administration. The results suggest that favipiravir causes toxic side effects in lung tissue even in healthy rats, implying potential pulmonary adverse effects that should be considered when using this drug therapeutically.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Erbaş et al., 9 Nov 2023, Turkey, peer-reviewed, 5 authors.
Contact: eliferbas4154@gmail.com.
Assessment of toxicological effects of favipiravir (T‐705) on the lung tissue of rats: An experimental study
Journal of Biochemical and Molecular Toxicology, doi:10.1002/jbt.23536
This study aimed to present new data on the side effects of favipiravir on healthy lung tissue and the respiratory system. In the study, two different durations (5 and 10 days) were preferred to determine the effect of favipiravir treatment due to clinical improvement rates of approximately 5 and 10 days during the use of favipiravir in COVID-19 patients. In addition, after 10 days of favipiravir treatment, animals were kept for 5 days without any treatment to determine the regeneration of lung tissues. Favipiravir was administered to rats by oral gavage at a daily dose of 200 mg/kg for 5 and 10 days, as in previous studies. At the end of the experiment, the histopathological and biochemical effects of favipiravir in the lung tissue were investigated. The data obtained from the study showed that favipiravir increased oxidative stress parameters, expression of apoptotic markers, and pro-inflammatory markers in lung tissue. Since malondialdehydes is an oxidant parameter, it increased in favipiravir-administered groups; It was determined that the antioxidant parameters glutathione, superoxide dismutase, glutathione peroxidase, and catalase decreased. Other markers used in the analysis are Bcl-2, Bax, NF-κB, interleukin (IL)-6, Muc1, iNOS, P2X7R, IL-6 and caspase-3. The levels of Bax, caspase-3, NF-κB, IL-6, Muc1, and P2X7R were increased in the Fav-treated groups compared with the control. However, the levels of Bcl-2 decreased in the Fav-treated groups. The present study proves that favipiravir, widely used today, causes side effects in lung tissue.
CONFLICT OF INTEREST STATEMENT The authors declare no conflict of interest.
References
Aebi, None, Methods Enzymol
Agrawal, Raju, Udwadia, None, Med. J. Armed Forces India
Albina, Cui, Mateo, Reichner, None, J. Immunol
Alderton, Cooper, Knowles, None, Biochem. J
Ali, Ibrahim, Burzangi, Ghoneim, Aljohani et al., None, Arabian J. Chem
Allen, Arjona, Santerre, Sawaya, None, All Life
Atcali, Yakut, Çağlayan, Ulucan, Kara, None, J. Exp. Clin. Med
Barberà-Cremades, Baroja-Mazo, Gomez, Machado, Di et al., None, FASEB. J
Bilici, Altuner, Süleyman, Bulut, Sarigul et al., None, Adv. Clin. Exp. Med
Burnstock, Fredholm, Verkhratsky, None, Curr. Top. Med. Chem
Chen, Jin, Narasaraju, Chen, Mcfarland et al., None, Biochem. Biophys. Res. Commun
Chen, Zhang, Huang, Yin, Cheng et al., None, Front. Pharmacol
Clarkson, Thompson, None, Am. J. Clin. Nutr
Conner, Grisham, None, Nutrition
Coutinho-Silva, Morandini, Savio, None, Biomed. J
Das, Santra, Lahiri, Guha Mazumder, None, Toxicol. Appl. Pharmacol
Desco, Asensi, Márquez, Martínez-Valls, Vento et al., None, Diabetes
Dhar, Ng, Dunne, Sutton, None, Virulence
Distefano, Fanzone, Palermo, Tiralongo, Cosentino et al., None, Diagnostics
Dolcet, Llobet, Pallares, Matias-Guiu, None, Virchows Arch
Downey, Elborn, None, J. Biochem. Mol. Toxicol
Doğan, Kaya, Demirel, Başeğmez, Şahin et al., None, Immunotoxicol
Driouich, Cochin, Lingas, Moureau, Touret et al., None, Nat. Commun
Duan, Hu, Li, Zhang, Jiang, None, Mol. Immunol
Er, Oliver, Cartron, Juin, Manon et al., None, Biochim. Biophys. Acta Bioenerget
Ergür, Yıldız, Şener, Kavurgacı, Ozturk, None, Sao Paulo Med. J
Erikson, Dobson, Dorman, Aschner, None, Sci. Total Environ
Evan, Vousden, None, Nature
Finberg, Ashraf, Julg, Ayoade, Marathe et al., None, Open Forum Infectious Diseases
Furuta, Gowen, Takahashi, Shiraki, Smee et al., None, Antiviral Res
Furuta, Takahashi, Fukuda, Kuno, Kamiyama et al., None, Antimicrob. Agents Chemother
Furuta, Takahashi, Shiraki, Sakamoto, Smee et al., None, Antiviral Res
Galam, Rajan, Failla, Soundararajan, Lockey et al., None, Am. J. Physiol. Lung Cell. Mol. Physiol
Goldhill, Velthuis, Fletcher, Langat, Zambon et al., None, Proc. Natl. Acad. Sci. U.S.A
Gunaydin-Akyildiz, Aksoy, Boran, Ilhan, Ozhan, None, Toxicol. Lett
He, Taylor, Fourgeaud, Bhattacharya, None, J. Neuroinflam
Heunks, Viña, Van Herwaarden, Folgering, Gimeno et al., None, Am. J. Physiol. Regul. Integr. Comp. Physiol
Hirano, None, Int. Immunol
Imai, Takase, Takeda, Kougo, None, Pediatr. Pulmonol
Inal, Kahraman, None, Toxicology
Irie, Nakagawa, Fujita, Tamura, Eto et al., None, Clin. Transl. Sci
Ishihara, Hirano, None, Cytokine Growth Factor Rev
Ishikawa, Hattori, Tanaka, Horimasu, Haruta et al., None, Respiration
Janks, Sprague, Egan, None, J. Immunol
Kaczmarek-Hajek, Zhang, Kopp, Grosche, Rissiek et al., None, eLife
Kalkan, Kapakin, Kara, Atabay, Karadeniz et al., None, J. Mol. Histol
Kara, Yakut, Caglayan, Atçalı, Ulucan et al., None, Drug Chem. Toxicol
Kardon, Price, Julian, Lagow, Tseng et al., None, Invest Ophthalmol. Visual Sci
Kato, Lillehoj, Kai, Kim, None, Front. Biosci
Kaur, Charan, Dutta, Sharma, Bhardwaj et al., None, Infect. Drug Resist
Kim, Hyun, Kim, Meerzaman, Lee et al., Cilia Mucus Mucocil, Interact
Kim, Lillehoj, None, Am. J. Respir. Cell Mol. Biol
Kopp, Krautloher, Ramírez-Fernández, Nicke, None, Front. Mol. Neurosci
Kubota, Haruta, None, J. Infect. Chemother
Kurt, Özmen, None, Cureus
Kyo, Kato, Park, Gajhate, Umehara et al., None, Am. J. Respir. Cell Mol. Biol
Lateef, Shakoor, Balk, None, Expert Opin. Drug Saf
Li, Dinwiddie, Harrod, Jiang, Kim, None, Am. J. Physiol. Lung Cell. Mol. Physiol
Li, Wang, Li, Zhu, Xu et al., None, Exp. Cell Res
Liang, Yan, Schor, None, Oncogene
Lillehoj, Hyun, Kim, Zhang, Lee et al., None, Am. J. Physiol. Lung Cell. Mol. Physiol
Liu, Garcia-Cardena, Sessa, None, Biochemistry
Lock, Eggert, Cooper, None, Clin. Chest Med
Lowry, Rosebrough, Farr, Randall, None, J. Biol. Chem
Martínez-García, Martínez-Banaclocha, Angosto-Bazarra, De Torre-Minguela, Baroja-Mazo et al., None, Nat. Commun
Mateos, Lecumberri, Ramos, Goya, Bravo, None, J. Chromatogr. B
Matkovics, Szabo, Varga, None, Laboratóriumi Diagnosztika
Mcauley, Corcilius, Tan, Payne, Mcguckin et al., None, Mucosal Immunol
Mcguckin, Lindén, Sutton, Florin, None, Nat. Rev. Microbiol
Mehrzadi, Fatemi, Esmaeilizadeh, Ghaznavi, Kalantar et al., None, Biomed. Pharmacother
Mendenhall, Russell, Juelich, Messina, Smee et al., None, Antimicrob. Agents Chemother
Mercan, None, Yüzüncü Yıl Üniversitesi Veteriner Fakültesi Dergisi
Metzger, Walser, Aprile-Garcia, Dedic, Chen et al., None, Purinergic Signal
Murai, Kawasuji, Takegoshi, Kaneda, Kimoto et al., None, Int. J. Infect. Dis
Musa, None, Arab J. Gastroenterol
Ohnishi, Yokoyama, Kondo, Hamada, Abe et al., None, Am. J. Respir. Crit. Care Med
Ohshimo, Bonella, Grammann, Starke, Cui et al., None, Sarcoid. Vascul. Diff. Lung Dis
Passmore, Byrne, Obonyo, See Hoe, Boon et al., None, Respir. Res
Pilkington, Pepperrell, Hill, None, J. Virus Erad
Placer, Cushman, Johnson, None, Anal. Biochem
Riteau, Gasse, Fauconnier, Gombault, Couegnat et al., None, Am. J. Respir. Crit. Care Med
Ryrfeldt, None, Toxicol. Lett
Samarghandian, Azimi-Nezhad, Farkhondeh, Samini, None, Biomed. Pharmacother
Samarghandian, Farkhondeh, Samini, Borji, None, Biochem. Res. Int
Savio, Andrade, De Andrade Mello, Santana, Moreira-Souza et al., None, Mol. Neurobiol
Savio, De Andrade Mello, Da Silva, Coutinho-Silva, None, Front. Pharmacol
Schottelius, Baldwin, None, Int. J. Colorecta. Dis
Schwaiblmair, Behr, Haeckel, Märkl, Foerg et al., None, Open Respir. Med. J
Sedlak, Lindsay, None, Anal. Biochem
Sharan Tripathi, Mishra, Shukla, Verma, Chaudhury et al., None, Arch. Toxicol
Sluyter, Stokes, None, Recent Patents DNA Gene Seq
Sun, Oberley, Li, None, Clin. Chem
Suttnar, Maova, Dyr, None, J. Chromatogr. B Biomed. Sci. Appl
Virgilio, Ben, Sarti, Giuliani, Falzoni, None, Immunity
Wang, Zhao, Wang, Liang, Yang et al., None, Int. Immunopharmacol
Warner, Paine, Christensen, Marletta, Richards et al., None, Am. J. Respir. Cell Mol. Biol
Yip, Woehrle, Corriden, Hirsh, Chen et al., None, FASEB. J
Zaidi, Banu, None, Clin. Chim. Acta
Çelik, Kandemir, Caglayan, Özdemir, Çomaklı et al., None, Mol. Biol. Rep
DOI record:
{
"DOI": "10.1002/jbt.23536",
"ISSN": [
"1095-6670",
"1099-0461"
],
"URL": "http://dx.doi.org/10.1002/jbt.23536",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:label /><jats:p>This study aimed to present new data on the side effects of favipiravir on healthy lung tissue and the respiratory system. In the study, two different durations (5 and 10 days) were preferred to determine the effect of favipiravir treatment due to clinical improvement rates of approximately 5 and 10 days during the use of favipiravir in COVID‐19 patients. In addition, after 10 days of favipiravir treatment, animals were kept for 5 days without any treatment to determine the regeneration of lung tissues. Favipiravir was administered to rats by oral gavage at a daily dose of 200 mg/kg for 5 and 10 days, as in previous studies. At the end of the experiment, the histopathological and biochemical effects of favipiravir in the lung tissue were investigated. The data obtained from the study showed that favipiravir increased oxidative stress parameters, expression of apoptotic markers, and pro‐inflammatory markers in lung tissue. Since malondialdehydes is an oxidant parameter, it increased in favipiravir‐administered groups; It was determined that the antioxidant parameters glutathione, superoxide dismutase, glutathione peroxidase, and catalase decreased. Other markers used in the analysis are Bcl‐2, Bax, NF‐κB, interleukin (IL)‐6, Muc1, iNOS, P2X7R, IL‐6 and caspase‐3. The levels of Bax, caspase‐3, NF‐κB, IL‐6, Muc1, and P2X7R were increased in the Fav‐treated groups compared with the control. However, the levels of Bcl‐2 decreased in the Fav‐treated groups. The present study proves that favipiravir, widely used today, causes side effects in lung tissue.</jats:p></jats:sec>",
"alternative-id": [
"10.1002/jbt.23536"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-10-19"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2023-09-01"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2023-11-09"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-1750-3889",
"affiliation": [
{
"name": "Department of Histology and Embryology Atatürk University Faculty of Veterinary Medicine Erzurum Turkey"
}
],
"authenticated-orcid": false,
"family": "Erbaş",
"given": "Elif",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Histology and Embryology Atatürk University Faculty of Veterinary Medicine Erzurum Turkey"
},
{
"name": "Department of Pharmacology Atatürk University Faculty of Medicine Erzurum Turkey"
}
],
"family": "Celep",
"given": "Nevra Aydemir",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Histology and Embryology Atatürk University Faculty of Veterinary Medicine Erzurum Turkey"
}
],
"family": "Tekiner",
"given": "Deniz",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biochemistry Bingöl University Faculty of Veterinary Medicine Bingöl Turkey"
}
],
"family": "Genç",
"given": "Aydın",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Histology and Embryology Atatürk University Faculty of Veterinary Medicine Erzurum Turkey"
}
],
"family": "Gedikli",
"given": "Semin",
"sequence": "additional"
}
],
"container-title": "Journal of Biochemical and Molecular Toxicology",
"container-title-short": "J Biochem & Molecular Tox",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2023,
11,
9
]
],
"date-time": "2023-11-09T11:34:36Z",
"timestamp": 1699529676000
},
"deposited": {
"date-parts": [
[
2023,
11,
9
]
],
"date-time": "2023-11-09T11:34:47Z",
"timestamp": 1699529687000
},
"indexed": {
"date-parts": [
[
2023,
11,
10
]
],
"date-time": "2023-11-10T00:43:33Z",
"timestamp": 1699577013217
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
11,
9
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
9
]
],
"date-time": "2023-11-09T00:00:00Z",
"timestamp": 1699488000000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jbt.23536",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2023,
11,
9
]
]
},
"published-online": {
"date-parts": [
[
2023,
11,
9
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1016/j.antiviral.2013.09.015",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_2_1"
},
{
"DOI": "10.1016/j.ajg.2020.03.002",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_3_1"
},
{
"DOI": "10.1128/AAC.01219-10",
"author": "Mendenhall M.",
"doi-asserted-by": "crossref",
"first-page": "782",
"issue": "2",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "e_1_2_9_4_1",
"volume": "55",
"year": "2011"
},
{
"DOI": "10.1073/pnas.1811345115",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_5_1"
},
{
"DOI": "10.1016/j.antiviral.2009.02.198",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_6_1"
},
{
"key": "e_1_2_9_7_1",
"unstructured": "S.Bilici D.Altuner Z.Süleyman S.Bulut C.Sarigul M.Gülaboğlu H.Süleyman Adv. Clin. Exp. Med.2023."
},
{
"DOI": "10.1517/14740338.4.4.723",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_8_1"
},
{
"DOI": "10.2174/1874306401206010063",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_9_1"
},
{
"DOI": "10.1016/S0378-4274(99)00281-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_10_1"
},
{
"DOI": "10.1378/chest.122.6_suppl.293S",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_11_1"
},
{
"DOI": "10.1016/S0272-5231(03)00129-1",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_12_1"
},
{
"DOI": "10.3390/diagnostics10040244",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_13_1"
},
{
"key": "e_1_2_9_14_1",
"unstructured": "M. F.Doğan K.Kaya H. H.Demirel M.Başeğmez Y.Şahin O.Çiftçi Immunotoxicol2023 1."
},
{
"DOI": "10.2147/IDR.S287934",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_15_1"
},
{
"DOI": "10.1016/S2055-6640(20)30016-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_16_1"
},
{
"DOI": "10.1111/cts.12827",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_17_1"
},
{
"DOI": "10.1016/j.arabjc.2021.103385",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_18_1"
},
{
"DOI": "10.1016/j.mjafi.2020.08.004",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_19_1"
},
{
"DOI": "10.1038/s41467-021-21992-w",
"author": "Driouich J.‐S.",
"doi-asserted-by": "crossref",
"first-page": "1735",
"issue": "1",
"journal-title": "Nat. Commun.",
"key": "e_1_2_9_20_1",
"volume": "12",
"year": "2021"
},
{
"author": "Finberg R. W.",
"key": "e_1_2_9_21_1",
"volume-title": "Open Forum Infectious Diseases",
"year": "2021"
},
{
"DOI": "10.1080/01480545.2022.2066116",
"author": "Kara A.",
"doi-asserted-by": "crossref",
"first-page": "546",
"journal-title": "Drug Chem. Toxicol.",
"key": "e_1_2_9_22_1",
"volume": "46",
"year": "2022"
},
{
"DOI": "10.1007/s11033-020-05302-z",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_23_1"
},
{
"DOI": "10.1016/S0021-9258(19)52451-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_24_1"
},
{
"DOI": "10.1016/0003-2697(68)90092-4",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_25_1"
},
{
"DOI": "10.1093/clinchem/34.3.497",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_26_1"
},
{
"author": "Matkovics B.",
"first-page": "248",
"journal-title": "Laboratóriumi Diagnosztika",
"key": "e_1_2_9_27_1",
"volume": "15",
"year": "1988"
},
{
"DOI": "10.1016/S0076-6879(84)05016-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_28_1"
},
{
"DOI": "10.1016/0003-2697(66)90167-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_29_1"
},
{
"DOI": "10.1186/s12931-018-0935-4",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_30_1"
},
{
"DOI": "10.1007/s10735-012-9412-4",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_31_1"
},
{
"key": "e_1_2_9_32_1",
"unstructured": "Y.Kurt Ö.Özmen Cureus2022 14(9)."
},
{
"DOI": "10.52142/omujecm.39.1.31",
"author": "Atcali T.",
"doi-asserted-by": "crossref",
"first-page": "156",
"issue": "1",
"journal-title": "J. Exp. Clin. Med.",
"key": "e_1_2_9_33_1",
"volume": "39",
"year": "2022"
},
{
"DOI": "10.1128/AAC.46.4.977-981.2002",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_34_1"
},
{
"DOI": "10.1080/26895293.2020.1835741",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_35_1"
},
{
"DOI": "10.3389/fphar.2021.683296",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_36_1"
},
{
"DOI": "10.1016/j.ijid.2021.03.048",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_37_1"
},
{
"DOI": "10.1590/1516-3180.2021.0489.r1.13082021",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_38_1"
},
{
"DOI": "10.1093/ajcn/72.2.637S",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_39_1"
},
{
"DOI": "10.1016/j.cccn.2003.11.003",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_40_1"
},
{
"DOI": "10.1016/j.biopha.2016.12.105",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_41_1"
},
{
"DOI": "10.1016/j.scitotenv.2004.04.044",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_42_1"
},
{
"DOI": "10.1016/j.biopha.2017.10.113",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_43_1"
},
{
"DOI": "10.1016/S0300-483X(00)00268-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_44_1"
},
{
"DOI": "10.1155/2016/2645237",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_45_1"
},
{
"author": "Mercan U.",
"first-page": "91",
"issue": "1",
"journal-title": "Yüzüncü Yıl Üniversitesi Veteriner Fakültesi Dergisi",
"key": "e_1_2_9_46_1",
"volume": "15",
"year": "2004"
},
{
"DOI": "10.1016/S0378-4347(00)00453-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_47_1"
},
{
"DOI": "10.1016/j.jchromb.2005.06.035",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_48_1"
},
{
"DOI": "10.1152/ajpregu.1999.277.6.R1697",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_49_1"
},
{
"DOI": "10.2337/diabetes.51.4.1118",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_50_1"
},
{
"DOI": "10.1016/j.toxlet.2022.09.011",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_51_1"
},
{
"DOI": "10.1038/35077213",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_52_1"
},
{
"DOI": "10.1016/j.bbabio.2006.05.032",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_53_1"
},
{
"DOI": "10.1038/sj.onc.1204815",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_54_1"
},
{
"DOI": "10.1007/s00428-005-1264-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_55_1"
},
{
"DOI": "10.1007/s003840050178",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_56_1"
},
{
"DOI": "10.1016/S0899-9007(96)00000-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_57_1"
},
{
"DOI": "10.1093/intimm/dxaa078",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_58_1"
},
{
"DOI": "10.1016/S1359-6101(02)00027-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_59_1"
},
{
"DOI": "10.1016/j.taap.2004.08.010",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_60_1"
},
{
"DOI": "10.1007/s00204-010-0559-z",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_61_1"
},
{
"DOI": "10.4049/jimmunol.1801101",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_62_1"
},
{
"DOI": "10.1016/j.bbrc.2004.05.048",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_63_1"
},
{
"DOI": "10.7554/eLife.36217",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_64_1"
},
{
"DOI": "10.1007/s11302-016-9546-z",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_65_1"
},
{
"DOI": "10.1186/s12974-017-0904-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_66_1"
},
{
"DOI": "10.2174/156802611795347627",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_67_1"
},
{
"DOI": "10.3389/fnmol.2019.00183",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_68_1"
},
{
"DOI": "10.2174/187221511794839219",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_69_1"
},
{
"DOI": "10.1016/j.immuni.2017.06.020",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_70_1"
},
{
"DOI": "10.1096/fj.12-205765",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_71_1"
},
{
"DOI": "10.4103/2319-4170.127803",
"author": "Coutinho‐Silva R.",
"doi-asserted-by": "crossref",
"first-page": "169",
"issue": "4",
"journal-title": "Biomed. J.",
"key": "e_1_2_9_72_1",
"volume": "37",
"year": "2014"
},
{
"DOI": "10.3389/fphar.2018.00052",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_73_1"
},
{
"DOI": "10.1038/s41467-019-10626-x",
"author": "Martínez‐García J. J.",
"doi-asserted-by": "crossref",
"first-page": "2711",
"issue": "1",
"journal-title": "Nat. Commun.",
"key": "e_1_2_9_74_1",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1007/s12035-016-0168-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_75_1"
},
{
"DOI": "10.1096/fj.08-126458",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_76_1"
},
{
"DOI": "10.1152/ajplung.00417.2015",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_77_1"
},
{
"DOI": "10.1016/j.molimm.2018.07.027",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_78_1"
},
{
"DOI": "10.1164/rccm.201003-0359OC",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_79_1"
},
{
"DOI": "10.1016/j.intimp.2015.04.035",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_80_1"
},
{
"DOI": "10.1016/j.yexcr.2018.03.002",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_81_1"
},
{
"DOI": "10.1038/nrmicro2538",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_82_1"
},
{
"key": "e_1_2_9_83_1",
"unstructured": "K. C.Kim S. W. Hyun B. T. Kim D. Meerzaman M. K. Lee E. P.Lillehoj Cilia Mucus Mucocil. Interact.2001 217."
},
{
"DOI": "10.1152/ajplung.2001.280.1.L181",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_84_1"
},
{
"author": "Kato K.",
"first-page": "68",
"journal-title": "Front. Biosci.",
"key": "e_1_2_9_85_1",
"volume": "2",
"year": "2010"
},
{
"DOI": "10.1165/rcmb.2008-0169TR",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_86_1"
},
{
"DOI": "10.1038/mi.2017.16",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_87_1"
},
{
"DOI": "10.1080/21505594.2017.1341021",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_88_1"
},
{
"DOI": "10.1152/ajplung.00225.2009",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_89_1"
},
{
"DOI": "10.1165/rcmb.2011-0142OC",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_90_1"
},
{
"author": "Kardon R.",
"first-page": "1328",
"issue": "7",
"journal-title": "Invest Ophthalmol. Visual Sci.",
"key": "e_1_2_9_91_1",
"volume": "40",
"year": "1999"
},
{
"DOI": "10.1002/ppul.10044",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_92_1"
},
{
"author": "Ohshimo S.",
"first-page": "47",
"issue": "1",
"journal-title": "Sarcoid. Vascul. Diff. Lung Dis.",
"key": "e_1_2_9_93_1",
"volume": "26",
"year": "2009"
},
{
"DOI": "10.1159/000324539",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_94_1"
},
{
"DOI": "10.1007/s10156-005-0416-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_95_1"
},
{
"DOI": "10.1164/ajrccm.165.3.2107134",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_96_1"
},
{
"DOI": "10.1042/bj3570593",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_97_1"
},
{
"DOI": "10.1021/bi00038a029",
"author": "Liu J.",
"doi-asserted-by": "crossref",
"issue": "38",
"journal-title": "Biochemistry",
"key": "e_1_2_9_98_1",
"volume": "34",
"year": "1995"
},
{
"DOI": "10.1165/ajrcmb.12.6.7539274",
"author": "Warner R. L.",
"doi-asserted-by": "crossref",
"first-page": "649",
"issue": "6",
"journal-title": "Am. J. Respir. Cell Mol. Biol.",
"key": "e_1_2_9_99_1",
"volume": "12",
"year": "1995"
},
{
"DOI": "10.4049/jimmunol.150.11.5080",
"author": "Albina J. E.",
"doi-asserted-by": "crossref",
"first-page": "5080",
"issue": "11",
"journal-title": "J. Immunol.",
"key": "e_1_2_9_100_1",
"volume": "150",
"year": "1993"
},
{
"DOI": "10.1002/(SICI)1096-9896(200002)190:2<115::AID-PATH491>3.0.CO;2-V",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_101_1"
}
],
"reference-count": 100,
"references-count": 100,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/jbt.23536"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Health, Toxicology and Mutagenesis",
"Toxicology",
"Molecular Biology",
"Molecular Medicine",
"Biochemistry",
"General Medicine"
],
"subtitle": [],
"title": "Assessment of toxicological effects of favipiravir (T‐705) on the lung tissue of rats: An experimental study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy"
}