Efficacy of Nigella sativa in COVID-19 patients: a systematic review and meta-analysis
et al., Journal of Emergency and Disaster Medicine, doi:10.1007/s44467-025-00004-7, PROSPERO CRD42023449783, Jan 2026
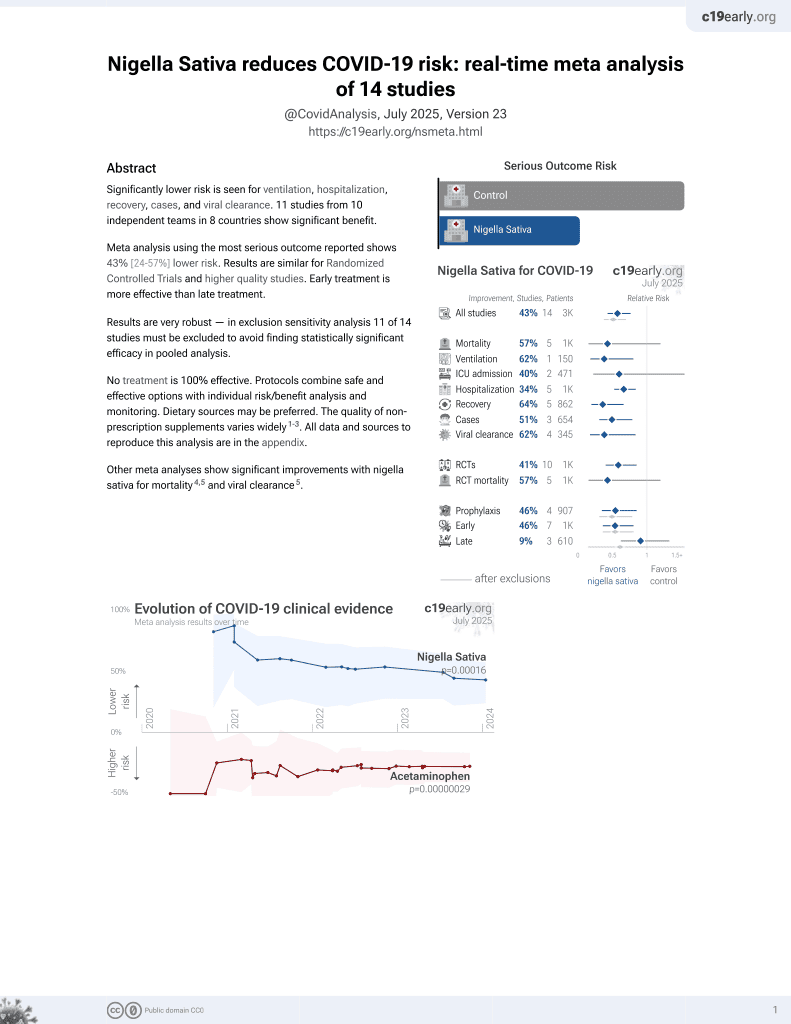
14th treatment shown to reduce risk in
January 2021, now with p = 0.00016 from 14 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
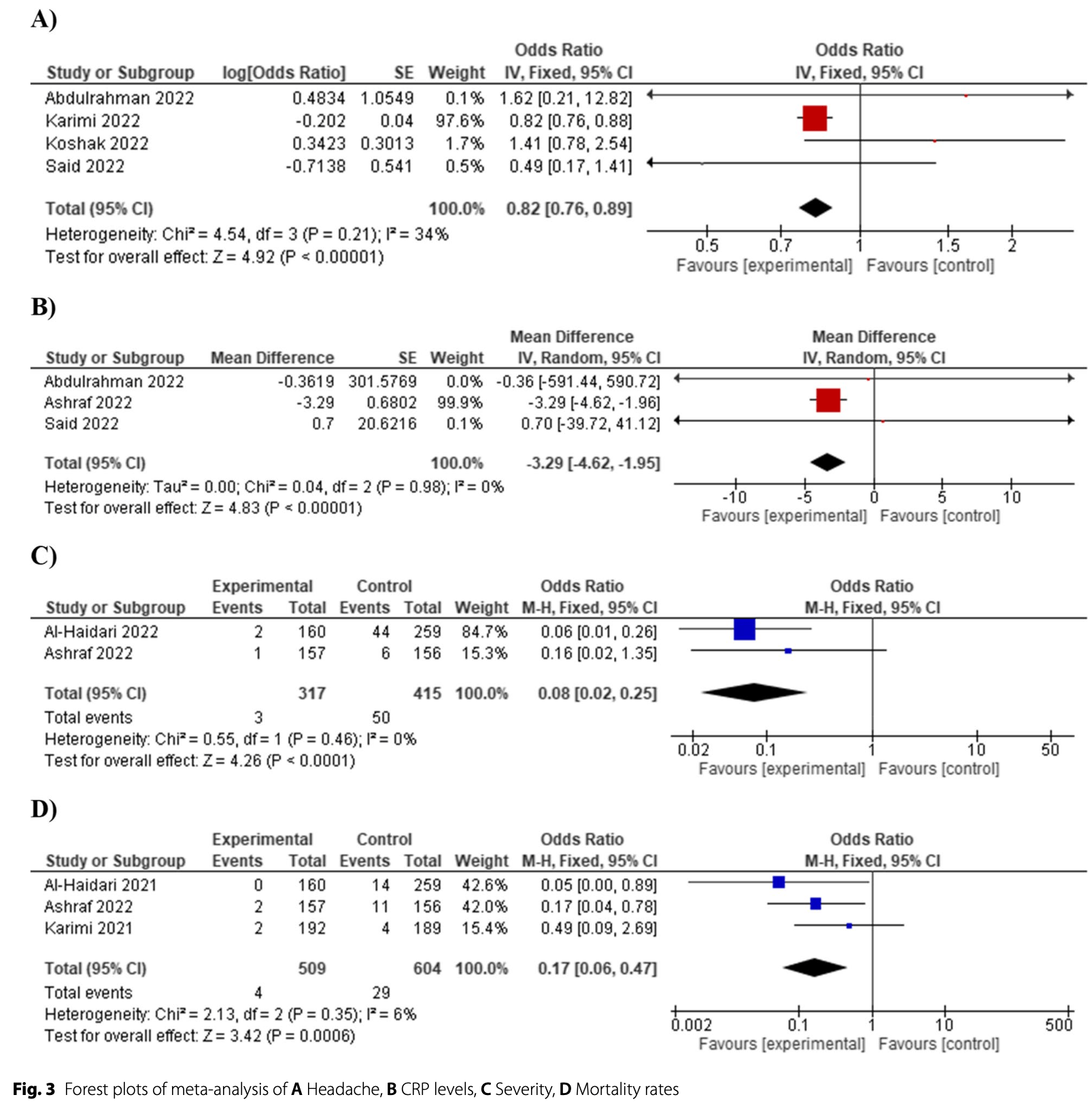
Systematic review and meta-analysis of 6 RCTs showing significantly lower mortality and symptom severity with nigella sativa treatment in 1,595 COVID-19 patients.
3 meta-analyses show significant improvements with nigella sativa for mortality1-3,
progression3, and
viral clearance2.
Currently there are 14 nigella sativa for COVID-19 studies, showing 57% lower mortality [-20‑85%], 62% lower ventilation [19‑82%], 40% lower ICU admission [-61‑78%], 34% lower hospitalization [16‑47%], and 51% fewer cases [21‑69%].
1.
Kow et al., The effect of Nigella sativa on the risk of mortality in patients with COVID‐19: A systematic review and meta‐analysis of randomized trials, Phytotherapy Research, doi:10.1002/ptr.7743.
Elrosasy et al., 28 Jan 2026, Egypt, peer-reviewed, 10 authors, trial PROSPERO CRD42023449783.
Contact: moaz_s_sayed@students.kasralainy.edu.eg.
Efficacy of Nigella sativa in COVID-19 patients: a systematic review and meta-analysis
Journal of Emergency and Disaster Medicine, doi:10.1007/s44467-025-00004-7
Background The COVID-19 pandemic has necessitated the investigation of potential treatment options to alleviate symptoms, reduce severe infection rates, and reduce mortality rates. Previous research on Nigella sativa, a phytomedicine with various therapeutic properties, has shown promise. This study aims to determine the efficacy of Nigella sativa as a treatment option for COVID-19 patients.
Methods We carried out a thorough systematic review of relevant literature from multiple databases until May 2024. Eligible studies were randomized controlled trials (RCTs) that investigated the efficacy of Nigella sativa as a treatment for COVID-19. We utilized the Cochrane Revised Risk of Bias 2 (ROB 2.0) to assess the quality of each included study. We carried out statistical analysis using RevMan version 5.4.
Results The meta-analysis included a total of six studies with a total of 1595 COVID-19 patients. The use of Nigella sativa significantly reduced the severity of infection symptoms (OR = 0.08, 95% CI [0.02 to 0.25], P < 0.0001) and demonstrated a significant reduction in mortality rates (P = 0.383). Also, it significantly reduced CRP levels (MD = -3.287, 95% CI [-4.620 to -1.955], P < 0.00001) and the overall likelihood of patients experiencing headache (OR = 0.82, 95% CI [0.76 to 0.89], P < 0.00001). Furthermore, in contrast, our study found no statistically significant effect of Nigella sativa on common COVID-19 symptoms such as fever, cough, fatigue, and ferritin levels, a key inflammatory marker. Similarly, it had no effect on the occurrence of rhinorrhea or shortness of breath among patients.
Conclusion This meta-analysis provides evidence that Nigella sativa is effective as a potential treatment option for COVID-19 patients. The use of this phytomedicine significantly reduces the likelihood of patients experiencing headaches and severe infection symptoms.
Supplementary Information The online version contains supplementary material available at h t t p s : / / d o i . o r g / 1 0 . 1 0 0 7 / s 4 4 4 6 7 -0 2 5 -0 0 0 0 4 -7. Supplementary Material 1.
Authors' contributions Conceptualization, Methodology: Amr Elrosasy; Project administration: Amira Mohamed Taha, Amr Elrosasy; Formal analysis and investigation: Amr Elrosasy; Data Curation: Rashad G. Mohamed, Islam Omar, Fatmaelzahraa Yasser Ali, Mohamed Elbanna; Writing-original draft preparation: Amira Mohamed Taha, Dang Nguyen, Nouran Ahmed Taha, Shirin Cadri,; Writing-review and editing: Amira Mohamed Taha, Moaz Elsayed Abouelmagd.
Funding None.
Data availability All data were extracted from published articles included in this review. The complete extraction sheet and analysis files (RevMan) are available from the corresponding author upon reasonable request.
Declarations Ethics approval and consent to participate Not applicable.
Competing interests The authors declare no competing interests.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Aljabre, Alakloby, Randhawa, Dermatological effects of Nigella sativa, J Dermatol Dermatol Surg, doi:10.1016/j.jdds.2015.04.002
Amin, Hosseinzadeh, Black cumin (Nigella sativa) and its active constituent, thymoquinone: an overview on the analgesic and anti-inflammatory effects, Planta Med, doi:10.1055/s-0035-1557838
Ashraf, Ashraf, Ashraf, Imran, Kalsoom et al., Honey and Nigella sativa against COVID-19 in Pakistan (HNS-COVID-PK): A multicenter placebo-controlled randomized clinical trial, Phytotherapy Research, doi:10.1002/ptr.7640
Barakat, Wakeel, Hagag, Effects of Nigella sativa on outcome of hepatitis C in Egypt, World J Gastroenterol, doi:10.3748/wjg.v19.i16.2529
Bin Abdulrahman, Bamosa, Bukhari, Siddiqui, Arafa et al., The effect of short treatment with Nigella sativa on symptoms, the cluster of differentiation (CD) profile, and inflammatory markers in mild COVID-19 patients: a randomized, doubleblind controlled trial, Int J Environ Res Public Health, doi:10.3390/ijerph191811798
Egger, Smith, Schneider, Minder, Bias in meta-analysis detected by a simple, graphical test, BMJ, doi:10.1136/bmj.315.7109.629
Hassan, Mabrouk, Shehata, Aboelhussein, Antineoplastic effects of bee honey and Nigella sativa on hepatocellular carcinoma cells, Integr Cancer Ther, doi:10.1177/1534735410387422
Higgins, Thomas, Chandler, Cumpston, Li, Cochrane handbook for systematic reviews of interventions (Version 6), Cochrane. h t t p s, doi:10.1002/9781119536604
Karimi, Zarei, Soleymani, Jamalimoghadamsiahkali, Asadi et al., Efficacy of Persian medicine herbal formulations (capsules and decoction) compared to standard care in patients with COVID-19, a multicenter open-labeled, randomized, controlled clinical trial, Phytotherapy Research, doi:10.1002/ptr.7277
Khazdair, Ghafari, Sadeghi, Possible therapeutic effects of Nigella sativa and its thymoquinone on COVID-19, Pharm Biol, doi:10.1080/13880209.2021.1931353
Kohandel, Farkhondeh, Aschner, Samarghandian, Anti-inflammatory effects of thymoquinone and its protective effects against several diseases, Biomedicine & Pharmacotherapy (Biomedecine & Pharmacotherapie), doi:10.1016/j.biopha.2021.111492
Kolayli, Kazaz, Özkök, Keskin, Kara et al., The phenolic composition, aroma compounds, physicochemical and antimicrobial properties of Nigella sativa L. (black cumin) honey, European Food Research and Technology = Zeitschrift Fur Lebensmittel-Untersuchung Und -Forschung A, doi:10.1007/s00217-022-04160-2
Koshak, Koshak, Mobeireek, Badawi, Wali et al., Nigella sativa for the treatment of COVID-19: an open-label randomized controlled clinical trial, Complement Ther Med, doi:10.1016/j.ctim.2021.102769
Koshak, Koshak, Nigella sativa L as a potential phytotherapy for coronavirus disease 2019: a mini review of in silico studies, Curr Ther Res Clin Exp, doi:10.1016/j.curtheres.2020.100602
Kow, Ramachandram, Hasan, The effect of Nigella sativa on the risk of mortality in patients with COVID-19: a systematic review and metaanalysis of randomized trials, Phytother Res, doi:10.1002/ptr.7743
Kulyar, Li, Mehmood, Waqas, Li et al., Potential influence of Nagella sativa (Black cumin) in reinforcing immune system: a hope to decelerate the COVID-19 pandemic, Phytomedicine, doi:10.1016/j.phymed.2020.153277
Lin, Hsu, Lin, Antiviral natural products and herbal medicines, J Tradit Complement Med, doi:10.4103/2225-4110.124335
Mahdavi, Namazi, Alizadeh, Farajnia, Nigella sativa oil with a calorie-restricted diet can improve biomarkers of systemic inflammation in obese women: a randomized double-blind, placebo-controlled clinical trial, J Clin Lipidol, doi:10.1016/j.jacl.2015.11.019
Mahmoud, Ameen, Effect of Nigella sativa and bee honey on pulmonary, hepatic and renal function in Sudanese in Khartoum state, Journal of Medicinal Plants Research, doi:10.5897/JMPR11.1357
Molla, Azad, Md, Azam, Hasib et al., A Review on Antiviral Effects of Nigella Sativa L Semantic Scholar 23
Nile, Nile, Qiu, Li, Jia et al., COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons, Cytokine Growth Factor Rev, doi:10.1016/j.cytogfr.2020.05.002
Ouzzani, Hammady, Fedorowicz, Elmagarmid, Rayyan-a web and mobile app for systematic reviews, Syst Rev, doi:10.1186/s13643-016-0384-4
Oyero, Toyama, Mitsuhiro, Onifade, Hidaka et al., Selective inhibition of hepatitis c virus replication by alpha-zam, a Nigella sativa seed formulation, Afr J Tradit Complement Altern Med, doi:10.21010/ajtcam.v13i6.20
Page, Bossuyt, Boutron, Hoffmann, Mulrow et al., The PRISMA 2020 statement: an updated guideline for reporting systematic reviews, BMJ (Clinical Research Ed, doi:10.1136/bmj.n71
Said, Abdulbaset, El-Kholy, Besckales, Sabri, The effect of Nigella sativa and vitamin D3 supplementation on the clinical outcome in COVID-19 patients: a randomized controlled clinical trial, Front Pharmacol, doi:10.3389/fphar.2022.1011522
Salem, Immunomodulatory and therapeutic properties of the Nigella sativa L. seed, Int Immunopharmacol, doi:10.1016/j.intimp.2005.06.008
Salem, Yar, Bamosa, Al-Quorain, Yasawy et al., Comparative study of Nigella sativa and triple therapy in eradication of Helicobacter pylori in patients with non-ulcer dyspepsia, Saudi J Gastroenterol, doi:10.4103/1319-3767.65201
Shad, Soubra, Cordato, The role of thymoquinone, a major constituent of Nigella sativa, in the treatment of inflammatory and infectious diseases, Clin Exp Pharmacol Physiol, doi:10.1111/1440-1681.13553
Shirvani, Rostamkhani, Arabzadeh, Mohammadi, Mohammadi, Potential role of Nigella sativa supplementation with physical activity in prophylaxis and treatment of COVID-19: a contemporary review, Sport Sci Health, doi:10.1007/s11332-021-00787-y
Sterne, Savović, Page, Elbers, Blencowe et al., RoB 2: a revised tool for assessing risk of bias in randomised trials, BMJ (Clinical Research Ed, doi:10.1136/bmj.l4898
Sytar, Brestic, Hajihashemi, Skalicky, Kubeš et al., COVID-19 prophylaxis efforts based on natural antiviral plant extracts and their compounds, Molecules. h t t, doi:10.3390/molecules26030727
Tariq, Nigella sativa seeds: folklore treatment in modern day medicine, Saudi J Gastroenterol, doi:10.4103/1319-3767.41725
Tavakkoli, Mahdian, Razavi, Hosseinzadeh, Review on clinical trials of Black seed (Nigella sativa) and its active constituent, thymoquinone, J Pharmacopuncture, doi:10.3831/KPI.2017.20.021
Taysi, Algburi, Mohammed, Ali, Taysi, Thymoquinone: a review on its pharmacological importance, and its association with oxidative stress, COVID-19, and radiotherapy, Mini Rev Med Chem, doi:10.2174/1389557522666220104151225
Toelzer, Gupta, Yadav, Borucu, Davidson et al., Free fatty acid binding pocket in the locked structure of SARS-CoV-2 spike protein, Science, doi:10.1126/science.abd3255
Ulasli, Gurses, Bayraktar, Yumrutas, Oztuzcu et al., The effects of Nigella sativa (Ns), Anthemis hyalina (Ah) and Citrus sinensis (Cs) extracts on the replication of coronavirus and the expression of TRP genes family, Mol Biol Rep, doi:10.1007/s11033-014-3019-7
Umar, Munir, Subhan, Azam, Un Nisa et al., Protective and antiviral activities of Nigella sativa against avian influenza (H9N2) in turkeys, Journal of the Saudi Society of Agricultural Sciences, doi:10.1016/j.jssas.2016.09.004
DOI record:
{
"DOI": "10.1007/s44467-025-00004-7",
"ISSN": [
"3059-4960"
],
"URL": "http://dx.doi.org/10.1007/s44467-025-00004-7",
"alternative-id": [
"4"
],
"article-number": "1",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "4 September 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Revised",
"name": "revised",
"order": 2,
"value": "18 October 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 3,
"value": "27 October 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 4,
"value": "28 January 2026"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Elrosasy",
"given": "Amr",
"sequence": "first"
},
{
"affiliation": [],
"family": "Taha",
"given": "Amira Mohamed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mohamed",
"given": "Rashad G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ali",
"given": "Fatmaelzahraa Yasser",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nguyen",
"given": "Dang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Taha",
"given": "Nouran Ahmed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cadri",
"given": "Shirin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Omar",
"given": "Islam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Elbanna",
"given": "Mohamed",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-0511-2429",
"affiliation": [],
"authenticated-orcid": false,
"family": "Abouelmagd",
"given": "Moaz Elsayed",
"sequence": "additional"
}
],
"container-title": "Journal of Emergency and Disaster Medicine",
"container-title-short": "J. Emerg. Disaster Med.",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2026,
1,
27
]
],
"date-time": "2026-01-27T23:02:52Z",
"timestamp": 1769554972000
},
"deposited": {
"date-parts": [
[
2026,
1,
27
]
],
"date-time": "2026-01-27T23:02:54Z",
"timestamp": 1769554974000
},
"indexed": {
"date-parts": [
[
2026,
1,
28
]
],
"date-time": "2026-01-28T13:03:46Z",
"timestamp": 1769605426068,
"version": "3.49.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2026,
1,
28
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2026,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2026,
1,
28
]
],
"date-time": "2026-01-28T00:00:00Z",
"timestamp": 1769558400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2026,
1,
28
]
],
"date-time": "2026-01-28T00:00:00Z",
"timestamp": 1769558400000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s44467-025-00004-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s44467-025-00004-7",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s44467-025-00004-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1007",
"published": {
"date-parts": [
[
2026,
1,
28
]
]
},
"published-online": {
"date-parts": [
[
2026,
1,
28
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/j.jdds.2015.04.002",
"author": "SHM Aljabre",
"doi-asserted-by": "publisher",
"first-page": "92",
"issue": "2",
"journal-title": "J Dermatol Dermatol Surg",
"key": "4_CR1",
"unstructured": "Aljabre SHM, Alakloby OM, Randhawa MA (2015) Dermatological effects of Nigella sativa. J Dermatol Dermatol Surg 19(2):92–98. https://doi.org/10.1016/j.jdds.2015.04.002",
"volume": "19",
"year": "2015"
},
{
"DOI": "10.1055/s-0035-1557838",
"author": "B Amin",
"doi-asserted-by": "publisher",
"first-page": "8",
"issue": "1–2",
"journal-title": "Planta Med",
"key": "4_CR2",
"unstructured": "Amin B, Hosseinzadeh H (2016) Black cumin (Nigella sativa) and its active constituent, thymoquinone: an overview on the analgesic and anti-inflammatory effects. Planta Med 82(1–2):8–16. https://doi.org/10.1055/s-0035-1557838",
"volume": "82",
"year": "2016"
},
{
"DOI": "10.1002/ptr.7640",
"doi-asserted-by": "publisher",
"key": "4_CR3",
"unstructured": "Ashraf S, Ashraf S, Ashraf M, Imran MA, Kalsoom L, Siddiqui UN, Farooq I, Akmal R, Akram MK, Ashraf S, Ghufran M, Majeed N, Habib Z, Rafique S, Abdin ZU, Arshad S, Shahab MS, Ahmad S, Zheng H, DOCTORS LOUNGE consortium (2023) Honey and Nigella sativa against COVID-19 in Pakistan (HNS-COVID-PK): A multicenter placebo-controlled randomized clinical trial. Phytotherapy Research. 37(2):627–644. https://doi.org/10.1002/ptr.7640"
},
{
"DOI": "10.3748/wjg.v19.i16.2529",
"author": "EMF Barakat",
"doi-asserted-by": "publisher",
"first-page": "2529",
"issue": "16",
"journal-title": "World J Gastroenterol",
"key": "4_CR4",
"unstructured": "Barakat EMF, El Wakeel LM, Hagag RS (2013) Effects of Nigella sativa on outcome of hepatitis C in Egypt. World J Gastroenterol 19(16):2529–2536. https://doi.org/10.3748/wjg.v19.i16.2529",
"volume": "19",
"year": "2013"
},
{
"DOI": "10.3390/ijerph191811798",
"author": "KA Bin Abdulrahman",
"doi-asserted-by": "publisher",
"journal-title": "Int J Environ Res Public Health",
"key": "4_CR5",
"unstructured": "Bin Abdulrahman KA, Bamosa AO, Bukhari AI, Siddiqui IA, Arafa MA, Mohsin AA, Althageel MF, Aljuaeed MO, Aldeailej IM, Alrajeh AI, Aldosari KM, Hawsawi NA, Zawbaee KI, Alsurayea SM (2022) The effect of short treatment with Nigella sativa on symptoms, the cluster of differentiation (CD) profile, and inflammatory markers in mild COVID-19 patients: a randomized, double-blind controlled trial. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph191811798",
"year": "2022"
},
{
"DOI": "10.37506/ijfmt.v15i3.15825",
"doi-asserted-by": "publisher",
"key": "4_CR6",
"unstructured": "Clinical trial of black seeds against COVID – 19 in kirkuk city/ iraq. (2021). Indian Journal of Forensic Medicine & Toxicology. https://doi.org/10.37506/ijfmt.v15i3.15825"
},
{
"key": "4_CR7",
"unstructured": "Coronavirus Death Rate (COVID-19) - Worldometer. (n.d.). Retrieved September 9, 2023, from https://www.worldometers.info/coronavirus/coronavirus-death-rate/#who-03-03-20"
},
{
"DOI": "10.1136/bmj.315.7109.629",
"author": "M Egger",
"doi-asserted-by": "publisher",
"first-page": "629",
"issue": "7109",
"journal-title": "BMJ",
"key": "4_CR8",
"unstructured": "Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634. https://doi.org/10.1136/bmj.315.7109.629",
"volume": "315",
"year": "1997"
},
{
"DOI": "10.1111/1440-1681.13553",
"author": "K Fatima Shad",
"doi-asserted-by": "publisher",
"first-page": "1445",
"issue": "11",
"journal-title": "Clin Exp Pharmacol Physiol",
"key": "4_CR9",
"unstructured": "Fatima Shad K, Soubra W, Cordato DJ (2021) The role of thymoquinone, a major constituent of Nigella sativa, in the treatment of inflammatory and infectious diseases. Clin Exp Pharmacol Physiol 48(11):1445–1453. https://doi.org/10.1111/1440-1681.13553",
"volume": "48",
"year": "2021"
},
{
"DOI": "10.1177/1534735410387422",
"author": "MI Hassan",
"doi-asserted-by": "publisher",
"first-page": "354",
"issue": "4",
"journal-title": "Integr Cancer Ther",
"key": "4_CR10",
"unstructured": "Hassan MI, Mabrouk GM, Shehata HH, Aboelhussein MM (2012) Antineoplastic effects of bee honey and Nigella sativa on hepatocellular carcinoma cells. Integr Cancer Ther 11(4):354–363. https://doi.org/10.1177/1534735410387422",
"volume": "11",
"year": "2012"
},
{
"DOI": "10.1002/9781119536604",
"doi-asserted-by": "publisher",
"key": "4_CR11",
"unstructured": "Higgins, J. P. T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., & Welch, V. A. (Eds.). (2019). Cochrane handbook for systematic reviews of interventions (Version 6). Cochrane. https://doi.org/10.1002/9781119536604"
},
{
"DOI": "10.1002/ptr.7277",
"doi-asserted-by": "publisher",
"key": "4_CR12",
"unstructured": "Karimi, M., Zarei, A., Soleymani, S., Jamalimoghadamsiahkali, S., Asadi, A., Shati, M., Jafari, M., Rezadoost, H., Kordafshar, G., Naghizadeh, A., Mardi, R., Namiranian, P., Khamechi, S. P., Ansari, N., Adel Mehraban, M. S., Aliakbarzadeh, H., Khanavi, M., Esmaealzadeh, N., Moravveji, A., … Zargaran, A. (2021). Efficacy of Persian medicine herbal formulations (capsules and decoction) compared to standard care in patients with COVID-19, a multicenter open-labeled, randomized, controlled clinical trial. Phytotherapy Research 35(11):6295–6309. https://doi.org/10.1002/ptr.7277"
},
{
"DOI": "10.1080/13880209.2021.1931353",
"author": "MR Khazdair",
"doi-asserted-by": "publisher",
"first-page": "696",
"issue": "1",
"journal-title": "Pharm Biol",
"key": "4_CR13",
"unstructured": "Khazdair MR, Ghafari S, Sadeghi M (2021) Possible therapeutic effects of Nigella sativa and its thymoquinone on COVID-19. Pharm Biol 59(1):696–703. https://doi.org/10.1080/13880209.2021.1931353",
"volume": "59",
"year": "2021"
},
{
"DOI": "10.1016/j.biopha.2021.111492",
"author": "Z Kohandel",
"doi-asserted-by": "publisher",
"journal-title": "Biomedicine & Pharmacotherapy (Biomedecine & Pharmacotherapie)",
"key": "4_CR14",
"unstructured": "Kohandel Z, Farkhondeh T, Aschner M, Samarghandian S (2021) Anti-inflammatory effects of thymoquinone and its protective effects against several diseases. Biomedicine & Pharmacotherapy (Biomedecine & Pharmacotherapie) 138:111492. https://doi.org/10.1016/j.biopha.2021.111492",
"volume": "138",
"year": "2021"
},
{
"DOI": "10.1007/s00217-022-04160-2",
"author": "S Kolayli",
"doi-asserted-by": "publisher",
"first-page": "653",
"issue": "3",
"journal-title": "European Food Research and Technology = Zeitschrift Fur Lebensmittel-Untersuchung Und -Forschung A",
"key": "4_CR15",
"unstructured": "Kolayli S, Kazaz G, Özkök A, Keskin M, Kara Y, Demir Kanbur E, Ertürk Ö (2023) The phenolic composition, aroma compounds, physicochemical and antimicrobial properties of Nigella sativa L. (black cumin) honey. European Food Research and Technology = Zeitschrift Fur Lebensmittel-Untersuchung Und -Forschung A 249(3):653–664. https://doi.org/10.1007/s00217-022-04160-2",
"volume": "249",
"year": "2023"
},
{
"DOI": "10.1016/j.ctim.2021.102769",
"author": "AE Koshak",
"doi-asserted-by": "publisher",
"journal-title": "Complement Ther Med",
"key": "4_CR16",
"unstructured": "Koshak AE, Koshak EA, Mobeireek AF, Badawi MA, Wali SO, Malibary HM, Atwah AF, Alhamdan MM, Almalki RA, Madani TA (2021) Nigella sativa for the treatment of COVID-19: an open-label randomized controlled clinical trial. Complement Ther Med 61:102769. https://doi.org/10.1016/j.ctim.2021.102769",
"volume": "61",
"year": "2021"
},
{
"DOI": "10.1016/j.curtheres.2020.100602",
"author": "DAE Koshak",
"doi-asserted-by": "publisher",
"journal-title": "Curr Ther Res Clin Exp",
"key": "4_CR17",
"unstructured": "Koshak DAE, Koshak PEA (2020) Nigella sativa L as a potential phytotherapy for coronavirus disease 2019: a mini review of in silico studies. Curr Ther Res Clin Exp 93:100602. https://doi.org/10.1016/j.curtheres.2020.100602",
"volume": "93",
"year": "2020"
},
{
"DOI": "10.1002/ptr.7743",
"author": "CS Kow",
"doi-asserted-by": "publisher",
"journal-title": "Phytother Res",
"key": "4_CR18",
"unstructured": "Kow CS, Ramachandram DS, Hasan SS (2023) The effect of Nigella sativa on the risk of mortality in patients with COVID-19: a systematic review and meta-analysis of randomized trials. Phytother Res. https://doi.org/10.1002/ptr.7743",
"year": "2023"
},
{
"DOI": "10.1016/j.phymed.2020.153277",
"author": "MF-E-A Kulyar",
"doi-asserted-by": "publisher",
"journal-title": "Phytomedicine",
"key": "4_CR19",
"unstructured": "Kulyar MF-E-A, Li R, Mehmood K, Waqas M, Li K, Li J (2021) Potential influence of Nagella sativa (Black cumin) in reinforcing immune system: a hope to decelerate the COVID-19 pandemic. Phytomedicine 85:153277. https://doi.org/10.1016/j.phymed.2020.153277",
"volume": "85",
"year": "2021"
},
{
"DOI": "10.4103/2225-4110.124335",
"author": "L-T Lin",
"doi-asserted-by": "publisher",
"first-page": "24",
"issue": "1",
"journal-title": "J Tradit Complement Med",
"key": "4_CR20",
"unstructured": "Lin L-T, Hsu W-C, Lin C-C (2014) Antiviral natural products and herbal medicines. J Tradit Complement Med 4(1):24–35. https://doi.org/10.4103/2225-4110.124335",
"volume": "4",
"year": "2014"
},
{
"DOI": "10.1016/j.jacl.2015.11.019",
"author": "R Mahdavi",
"doi-asserted-by": "publisher",
"first-page": "1203",
"issue": "5",
"journal-title": "J Clin Lipidol",
"key": "4_CR21",
"unstructured": "Mahdavi R, Namazi N, Alizadeh M, Farajnia S (2016) Nigella sativa oil with a calorie-restricted diet can improve biomarkers of systemic inflammation in obese women: a randomized double-blind, placebo-controlled clinical trial. J Clin Lipidol 10(5):1203–1211. https://doi.org/10.1016/j.jacl.2015.11.019",
"volume": "10",
"year": "2016"
},
{
"key": "4_CR22",
"unstructured": "Molla, S., Azad, M., Md Ali Azam Al Hasib, Hossain, M. M., Md, Ahammed, S., Rana, S., & Islam, M. T. (2019). [PDF] A Review on Antiviral Effects of Nigella Sativa L Semantic Scholar"
},
{
"key": "4_CR23",
"unstructured": "Morton SC, Adams JL, Suttorp MJ, et al. Meta-regression Approaches: What, Why, When, and How? Rockville (MD): Agency for Healthcare Research and Quality (US); 2004 Mar. (Technical Reviews, No. 8.) 1, Introduction. Available from: https://www.ncbi.nlm.nih.gov/books/NBK43897/"
},
{
"DOI": "10.5897/JMPR11.1357",
"doi-asserted-by": "publisher",
"key": "4_CR24",
"unstructured": "Nahid Mahmoud AL Ameen. (2011). Effect of Nigella sativa and bee honey on pulmonary, hepatic and renal function in Sudanese in Khartoum state. Journal of Medicinal Plants Research. 5(31). https://doi.org/10.5897/JMPR11.1357"
},
{
"DOI": "10.1016/j.cytogfr.2020.05.002",
"author": "SH Nile",
"doi-asserted-by": "publisher",
"first-page": "66",
"journal-title": "Cytokine Growth Factor Rev",
"key": "4_CR25",
"unstructured": "Nile SH, Nile A, Qiu J, Li L, Jia X, Kai G (2020) COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev 53:66–70. https://doi.org/10.1016/j.cytogfr.2020.05.002",
"volume": "53",
"year": "2020"
},
{
"DOI": "10.1186/s13643-016-0384-4",
"author": "M Ouzzani",
"doi-asserted-by": "publisher",
"first-page": "210",
"issue": "1",
"journal-title": "Syst Rev",
"key": "4_CR26",
"unstructured": "Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan-a web and mobile app for systematic reviews. Syst Rev 5(1):210. https://doi.org/10.1186/s13643-016-0384-4",
"volume": "5",
"year": "2016"
},
{
"DOI": "10.21010/ajtcam.v13i6.20",
"author": "OG Oyero",
"doi-asserted-by": "publisher",
"first-page": "144",
"issue": "6",
"journal-title": "Afr J Tradit Complement Altern Med",
"key": "4_CR27",
"unstructured": "Oyero OG, Toyama M, Mitsuhiro N, Onifade AA, Hidaka A, Okamoto M, Baba M (2016) Selective inhibition of hepatitis c virus replication by alpha-zam, a Nigella sativa seed formulation. Afr J Tradit Complement Altern Med 13(6):144–148. https://doi.org/10.21010/ajtcam.v13i6.20",
"volume": "13",
"year": "2016"
},
{
"DOI": "10.1136/bmj.n71",
"doi-asserted-by": "publisher",
"key": "4_CR28",
"unstructured": "Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., … Moher, D. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Research Ed.). 372:n71. https://doi.org/10.1136/bmj.n71"
},
{
"DOI": "10.3389/fphar.2022.1011522",
"author": "SA Said",
"doi-asserted-by": "publisher",
"first-page": "1011522",
"journal-title": "Front Pharmacol",
"key": "4_CR29",
"unstructured": "Said SA, Abdulbaset A, El-Kholy AA, Besckales O, Sabri NA (2022) The effect of Nigella sativa and vitamin D3 supplementation on the clinical outcome in COVID-19 patients: a randomized controlled clinical trial. Front Pharmacol 13:1011522. https://doi.org/10.3389/fphar.2022.1011522",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.4103/1319-3767.65201",
"author": "EM Salem",
"doi-asserted-by": "publisher",
"first-page": "207",
"issue": "3",
"journal-title": "Saudi J Gastroenterol",
"key": "4_CR30",
"unstructured": "Salem EM, Yar T, Bamosa AO, Al-Quorain A, Yasawy MI, Alsulaiman RM, Randhawa MA (2010) Comparative study of Nigella sativa and triple therapy in eradication of Helicobacter pylori in patients with non-ulcer dyspepsia. Saudi J Gastroenterol 16(3):207–214. https://doi.org/10.4103/1319-3767.65201",
"volume": "16",
"year": "2010"
},
{
"DOI": "10.1016/j.intimp.2005.06.008",
"author": "ML Salem",
"doi-asserted-by": "publisher",
"first-page": "1749",
"issue": "13–14",
"journal-title": "Int Immunopharmacol",
"key": "4_CR31",
"unstructured": "Salem ML (2005) Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol 5(13–14):1749–1770. https://doi.org/10.1016/j.intimp.2005.06.008",
"volume": "5",
"year": "2005"
},
{
"DOI": "10.1007/s11332-021-00787-y",
"author": "H Shirvani",
"doi-asserted-by": "publisher",
"first-page": "849",
"issue": "4",
"journal-title": "Sport Sci Health",
"key": "4_CR32",
"unstructured": "Shirvani H, Rostamkhani F, Arabzadeh E, Mohammadi F, Mohammadi F (2021) Potential role of Nigella sativa supplementation with physical activity in prophylaxis and treatment of COVID-19: a contemporary review. Sport Sci Health 17(4):849–854. https://doi.org/10.1007/s11332-021-00787-y",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.1136/bmj.l4898",
"doi-asserted-by": "publisher",
"key": "4_CR33",
"unstructured": "Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., Cates, C. J., Cheng, H.-Y., Corbett, M. S., Eldridge, S. M., Emberson, J. R., Hernán, M. A., Hopewell, S., Hróbjartsson, A., Junqueira, D. R., Jüni, P., Kirkham, J. J., Lasserson, T., Li, T., … Higgins, J. P. T. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical Research Ed.). 366;l4898. https://doi.org/10.1136/bmj.l4898"
},
{
"DOI": "10.3390/molecules26030727",
"author": "O Sytar",
"doi-asserted-by": "publisher",
"journal-title": "Molecules",
"key": "4_CR34",
"unstructured": "Sytar O, Brestic M, Hajihashemi S, Skalicky M, Kubeš J, Lamilla-Tamayo L, Ibrahimova U, Ibadullayeva S, Landi M (2021) COVID-19 prophylaxis efforts based on natural antiviral plant extracts and their compounds. Molecules. https://doi.org/10.3390/molecules26030727",
"year": "2021"
},
{
"DOI": "10.4103/1319-3767.41725",
"author": "M Tariq",
"doi-asserted-by": "publisher",
"first-page": "105",
"issue": "3",
"journal-title": "Saudi J Gastroenterol",
"key": "4_CR35",
"unstructured": "Tariq M (2008) Nigella sativa seeds: folklore treatment in modern day medicine. Saudi J Gastroenterol 14(3):105–106. https://doi.org/10.4103/1319-3767.41725",
"volume": "14",
"year": "2008"
},
{
"DOI": "10.3831/KPI.2017.20.021",
"author": "A Tavakkoli",
"doi-asserted-by": "publisher",
"first-page": "179",
"issue": "3",
"journal-title": "J Pharmacopuncture",
"key": "4_CR36",
"unstructured": "Tavakkoli A, Mahdian V, Razavi BM, Hosseinzadeh H (2017) Review on clinical trials of Black seed (Nigella sativa) and its active constituent, thymoquinone. J Pharmacopuncture 20(3):179–193. https://doi.org/10.3831/KPI.2017.20.021",
"volume": "20",
"year": "2017"
},
{
"DOI": "10.2174/1389557522666220104151225",
"author": "S Taysi",
"doi-asserted-by": "publisher",
"first-page": "1847",
"issue": "14",
"journal-title": "Mini Rev Med Chem",
"key": "4_CR37",
"unstructured": "Taysi S, Algburi FS, Mohammed ZR, Ali OA, Taysi ME (2022) Thymoquinone: a review on its pharmacological importance, and its association with oxidative stress, COVID-19, and radiotherapy. Mini Rev Med Chem 22(14):1847–1875. https://doi.org/10.2174/1389557522666220104151225",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1126/science.abd3255",
"author": "C Toelzer",
"doi-asserted-by": "publisher",
"first-page": "725",
"issue": "6517",
"journal-title": "Science",
"key": "4_CR38",
"unstructured": "Toelzer C, Gupta K, Yadav SKN, Borucu U, Davidson AD, Kavanagh Williamson M, Shoemark DK, Garzoni F, Staufer O, Milligan R, Capin J, Mulholland AJ, Spatz J, Fitzgerald D, Berger I, Schaffitzel C (2020) Free fatty acid binding pocket in the locked structure of SARS-CoV-2 spike protein. Science 370(6517):725–730. https://doi.org/10.1126/science.abd3255",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1007/s11033-014-3019-7",
"author": "M Ulasli",
"doi-asserted-by": "publisher",
"first-page": "1703",
"issue": "3",
"journal-title": "Mol Biol Rep",
"key": "4_CR39",
"unstructured": "Ulasli M, Gurses SA, Bayraktar R, Yumrutas O, Oztuzcu S, Igci M, Igci YZ, Cakmak EA, Arslan A (2014) The effects of Nigella sativa (Ns), Anthemis hyalina (Ah) and Citrus sinensis (Cs) extracts on the replication of coronavirus and the expression of TRP genes family. Mol Biol Rep 41(3):1703–1711. https://doi.org/10.1007/s11033-014-3019-7",
"volume": "41",
"year": "2014"
},
{
"DOI": "10.1016/j.jssas.2016.09.004",
"doi-asserted-by": "publisher",
"key": "4_CR40",
"unstructured": "Umar, S., Munir, M. T., Subhan, S., Azam, T., un Nisa, Q., Khan, M. I., Umar, W., ur Rehman, Z., Saqib, A. S., & Shah, M. A. (2016). Protective and antiviral activities of Nigella sativa against avian influenza (H9N2) in turkeys. Journal of the Saudi Society of Agricultural Sciences. https://doi.org/10.1016/j.jssas.2016.09.004"
}
],
"reference-count": 40,
"references-count": 40,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s44467-025-00004-7"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy of Nigella sativa in COVID-19 patients: a systematic review and meta-analysis",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "2"
}