Honey and Nigella sativa against COVID-19 in Pakistan (HNS-COVID-PK): A multi-center placebo-controlled randomized clinical trial
et al., Phytotherapy Research, doi:10.1002/ptr.7640, HNS-COVID-PK, NCT04347382, Nov 2020 (preprint)
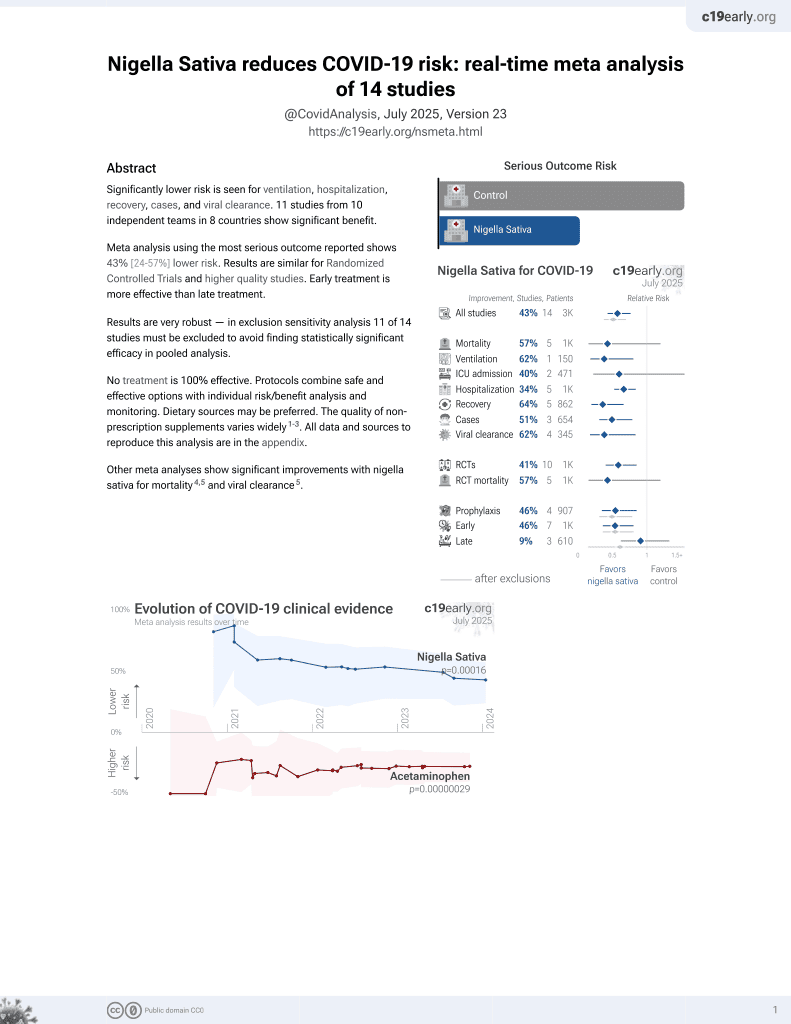
14th treatment shown to reduce risk in
January 2021, now with p = 0.00016 from 14 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
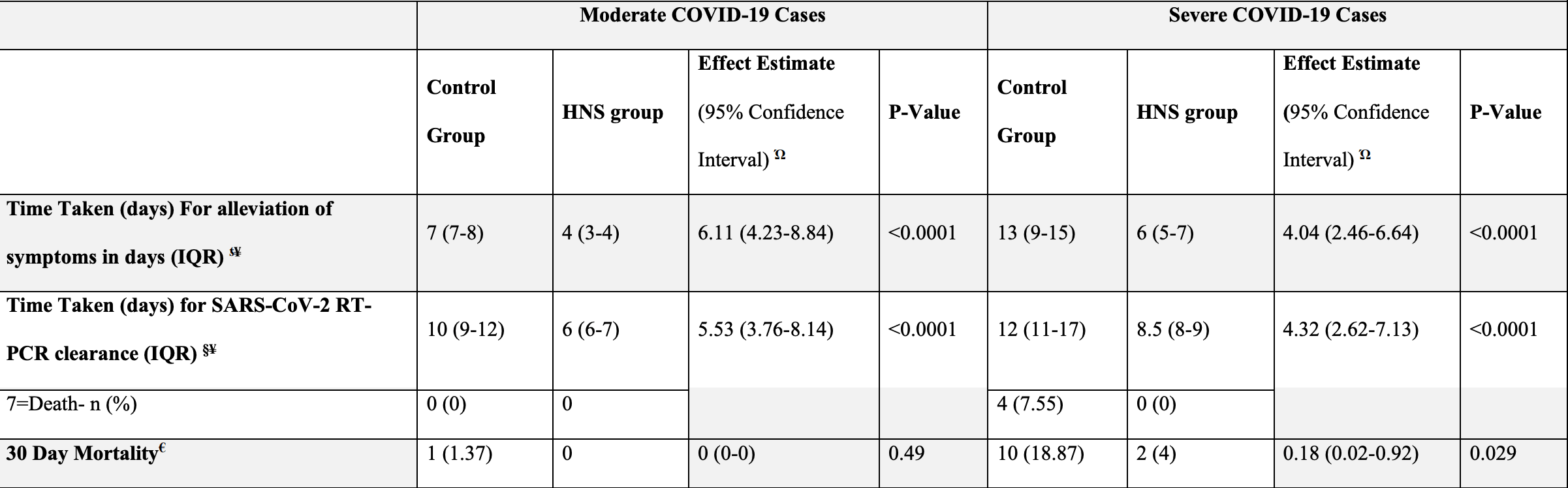
RCT with 157 patients treated with honey and nigella sativa, and 156 control patients, showing significantly faster recovery and viral clearance.
Honey (1gm/kg/day) plus encapsulated nigella sativa seeds (80mg/kg/day) orally in 2-3 divided doses daily for up to 13 days.
|
risk of death, 81.9% lower, RR 0.18, p = 0.01, treatment 2 of 157 (1.3%), control 11 of 156 (7.1%), NNT 17, all cases.
|
|
risk of death, 67.1% lower, RR 0.33, p = 0.49, treatment 0 of 107 (0.0%), control 1 of 103 (1.0%), NNT 103, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), moderate cases.
|
|
risk of death, 78.8% lower, RR 0.21, p = 0.03, treatment 2 of 50 (4.0%), control 10 of 53 (18.9%), NNT 6.7, severe cases.
|
|
risk of no recovery, 83.6% lower, HR 0.16, p < 0.001, treatment 107, control 103, inverted to make HR<1 favor treatment, moderate cases.
|
|
risk of no recovery, 75.2% lower, HR 0.25, p < 0.001, treatment 50, control 53, inverted to make HR<1 favor treatment, severe cases.
|
|
risk of no viral clearance, 81.9% lower, HR 0.18, p < 0.001, treatment 107, control 103, inverted to make HR<1 favor treatment, moderate cases.
|
|
risk of no viral clearance, 76.9% lower, HR 0.23, p < 0.001, treatment 50, control 53, inverted to make HR<1 favor treatment, severe cases.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Ashraf et al., 3 Nov 2020, Randomized Controlled Trial, placebo-controlled, Pakistan, peer-reviewed, 29 authors, study period 30 April, 2020 - 29 July, 2020, this trial uses multiple treatments in the treatment arm (combined with honey) - results of individual treatments may vary, trial NCT04347382 (history) (HNS-COVID-PK).
Abstract: medRxiv preprint doi: https://doi.org/10.1101/2020.10.30.20217364; this version posted November 30, 2020. The copyright holder for this
preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
It is made available under a CC-BY 4.0 International license .
Honey and Nigella sativa against COVID-19 in Pakistan (HNS-COVID-PK):
A multi-center placebo-controlled randomized clinical trial
Sohaib Ashraf1,2, Shoaib Ashraf 2, Moneeb Ashraf3, Muhammad Ahmad Imran3, Larab
Kalsoom3, Uzma Nasim Siddiqui3, Iqra Farooq3, Zaighum Habib, Sidra Ashraf, Muhammad
Ghufran, Muhammad Kiwan Akram, Nighat Majeed, Zain-ul-Abdin, Rutaba Akmal, Sundas
Rafique, Khawar Nawaz, Muhammad Ismail K Yousaf, Sohail Ahmad, Muhammad Sarmad
Shahab, Muhammad Faisal Nadeem, Muhammad Azam, Hui Zheng, Amber Malik, Mahmood
Ayyaz, Talha Mahmud, Qazi Abdul Saboor, Ali Ahmad, Muhammad Ashraf, Mateen Izhar for
the COALITION COVID-19 Shaikh Zayed Ω
1 Correspondence
to:
Dr Sohaib Ashraf: Department of Cardiology, Shaikh Zayed Post-Graduate Medical
Complex, Lahore, 54600, Pakistan.
sohaib-ashraf@outlook.com
2 Joint
3
First Author
Joint Second Author
NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice.
medRxiv preprint doi: https://doi.org/10.1101/2020.10.30.20217364; this version posted November 30, 2020. The copyright holder for this
preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
It is made available under a CC-BY 4.0 International license .
Ω COALITION COVID-19 Shaikh Zayed:
Abubakar Hilal, Arz Muhammad, Zeeshan Shaukat, Ayesha Khaqan, Kanwal Hayat, Shahroze Arshad,
Muhammad Hassan, Abeer-bin-Awais, Ammara Ahmad, Tayyab Mughal, Abdur Rehman Virk,
Muhammad Umer, Muhammad Suhail, Sibgha Zulfiqar, Saulat Sarfraz, Muhammad Imran Anwar,
Ayesha Humayun
Affiliations:
1. Department of Cardiology, Shaikh Zayed Post-Graduate Medical Complex, Lahore,
Pakistan.
i. S Ashraf, MBBS
ii. Z U Abdin, MBBS
iii. A Hilal, MBBS
iv. A Muhammad, MBBS
v. Z Shaukat, MBBS
vi. A Khaqan, MBBS
vii. K Hayat, M.Phil
viii. Prof. Q A Saboor
2. Department of Pathobiology, Riphah University, Lahore, Pakistan
i. Sh Ashraf, PhD
3. Department of Pharmacology, Kingedward Medical University, Mayo Hospital, Lahore,
Pakistan.
i. M Ashraf, MBBS
4. Department of Microbiology, Shaikh Zayed Post-Graduate Medical Institute, Lahore,
Pakistan.
i. M A Imran, MBBS
medRxiv preprint doi: https://doi.org/10.1101/2020.10.30.20217364; this version posted November 30, 2020. The copyright holder for this
preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
It is made available under a CC-BY 4.0 International license .
ii. Prof. M Izhar, PhD
5. Division of Telemedicine, Doctor’s Lounge, Lahore, Pakistan.
i. M A Imran, MBBS
ii. M S Shahab, MBBS
iii. I Farooq, MBBS
iv. S Rafique, MBBS
6. Department of Internal Medicine, Services Institute of Medical Sciences, Lahore,
Pakistan.
i. L Kalsoom, MBBS
ii. N Majeed, MBBS
7. Department of Medicine, Port Macquarie Base Hospital, New South Wales, Australia.
i. U N Siddique, MBBS
8. Department of Internal Medicine, Shaikh Zayed Post-Graduate Medical Institute, Lahore,
Pakistan.
i. S Arshad, MBBS
ii. M Hassan, MBBS
iii. U N Siddique, MBBS
9...
DOI record:
{
"DOI": "10.1002/ptr.7640",
"ISSN": [
"0951-418X",
"1099-1573"
],
"URL": "http://dx.doi.org/10.1002/ptr.7640",
"alternative-id": [
"10.1002/ptr.7640"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-01-03"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-09-18"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-11-24"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-3127-3557",
"affiliation": [
{
"name": "Department of Cardiology Shaikh Zayed Post‐Graduate Medical Institute Lahore Pakistan"
}
],
"authenticated-orcid": false,
"family": "Ashraf",
"given": "Sohaib",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-1218-4877",
"affiliation": [
{
"name": "Department of Pathobiology Riphah University Lahore Pakistan"
}
],
"authenticated-orcid": false,
"family": "Ashraf",
"given": "Shoaib",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5769-9524",
"affiliation": [
{
"name": "Department of Pharmacology King Edward Medical University, Mayo Hospital Lahore Pakistan"
}
],
"authenticated-orcid": false,
"family": "Ashraf",
"given": "Moneeb",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3358-229X",
"affiliation": [
{
"name": "Department of Microbiology Shaikh Zayed Post‐Graduate Medical Institute Lahore Pakistan"
}
],
"authenticated-orcid": false,
"family": "Imran",
"given": "Muhammad Ahmad",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1075-6285",
"affiliation": [
{
"name": "Department of Internal Medicine Services Institute of Medical Sciences Lahore Pakistan"
}
],
"authenticated-orcid": false,
"family": "Kalsoom",
"given": "Larab",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1004-5689",
"affiliation": [
{
"name": "Department of Medicine Port Macquarie Base Hospital Port Macquarie New South Wales Australia"
},
{
"name": "Department of Internal Medicine Shaikh Zayed Post‐Graduate Medical Institute Lahore Pakistan"
}
],
"authenticated-orcid": false,
"family": "Siddiqui",
"given": "Uzma N.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9832-9589",
"affiliation": [
{
"name": "Department of Pediatrics Surgery Children Hospital Lahore Pakistan"
}
],
"authenticated-orcid": false,
"family": "Farooq",
"given": "Iqra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine Sahara Medical College Narowal Pakistan"
}
],
"family": "Akmal",
"given": "Rutaba",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8415-9363",
"affiliation": [
{
"name": "Department of Nutrition University of Veterinary and Animal Sciences Lahore Pakistan"
}
],
"authenticated-orcid": false,
"family": "Akram",
"given": "Muhammad Kiwan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biochemistry University of Veterinary and Animal Sciences Lahore Pakistan"
}
],
"family": "Ashraf",
"given": "Sidra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medico Cirujano ESACHS (Empresa de Servico Externo de la Asociacion Chilena de Seguridad) Santiago Chile"
}
],
"family": "Ghufran",
"given": "Muhammad",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0238-7520",
"affiliation": [
{
"name": "Department of Internal Medicine Services Institute of Medical Sciences Lahore Pakistan"
}
],
"authenticated-orcid": false,
"family": "Majeed",
"given": "Nighat",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Orthopedics Shaikh Zayed Post‐Graduate Medical Complex Lahore Pakistan"
}
],
"family": "Habib",
"given": "Zaighum",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Oncology Mayo Hospital, King Edward Medical University Lahore Pakistan"
}
],
"family": "Rafique",
"given": "Sundas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiology Shaikh Zayed Post‐Graduate Medical Institute Lahore Pakistan"
}
],
"family": "‐Abdin",
"given": "Zain‐ul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine Shaikh Zayed Post‐Graduate Medical Institute Lahore Pakistan"
}
],
"family": "Arshad",
"given": "Shahroze",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine Allied Hospital, Faisalabad Medical University Faisalabad Pakistan"
}
],
"family": "Shahab",
"given": "Muhammad Sarmad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Poultry Production University of Veterinary and Animal Sciences Lahore Pakistan"
}
],
"family": "Ahmad",
"given": "Sohail",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine Massachusetts General Hospital, Harvard Medical School Boston Massachusetts USA"
}
],
"family": "Zheng",
"given": "Hui",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Plastic Surgery Shaikh Zayed Post‐Graduate Medical Institute Lahore Pakistan"
}
],
"family": "Mirza",
"given": "Ali Rafique",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Physiology Shaikh Khalifa Bin Zayed Al‐Nahyan Medical and Dental College Lahore Pakistan"
}
],
"family": "Zulfiqar",
"given": "Sibgha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of General Surgery Shaikh Zayed Post‐Graduate Medical Institute Lahore Pakistan"
}
],
"family": "Anwar",
"given": "Muhamad Imran",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7992-8765",
"affiliation": [
{
"name": "Department of Public Health and Community Medicine Shaikh Zayed Postgraduate Medical Institute Lahore Pakistan"
}
],
"authenticated-orcid": false,
"family": "Humayun",
"given": "Ayesha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pulmonology Shaikh Zayed Post‐Graduate Medical Institute Lahore Pakistan"
}
],
"family": "Mahmud",
"given": "Talha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiology Shaikh Zayed Post‐Graduate Medical Institute Lahore Pakistan"
}
],
"family": "Saboor",
"given": "Qazi Abdul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Microbiology, Infectious Diseases & Immunology Centre Hospitalier Universitaire (CHU) Sainte Justine/University of Montreal Montreal Quebec Canada"
}
],
"family": "Ahmad",
"given": "Ali",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacology and Toxicology University of Veterinary and Animal Sciences Lahore Pakistan"
}
],
"family": "Ashraf",
"given": "Muhammad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Microbiology Shaikh Zayed Post‐Graduate Medical Institute Lahore Pakistan"
}
],
"family": "Izhar",
"given": "Mateen",
"sequence": "additional"
},
{
"affiliation": [],
"name": "DOCTORS LOUNGE consortium",
"sequence": "additional"
}
],
"container-title": "Phytotherapy Research",
"container-title-short": "Phytotherapy Research",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2022,
11,
24
]
],
"date-time": "2022-11-24T15:20:47Z",
"timestamp": 1669303247000
},
"deposited": {
"date-parts": [
[
2022,
11,
24
]
],
"date-time": "2022-11-24T15:21:00Z",
"timestamp": 1669303260000
},
"indexed": {
"date-parts": [
[
2022,
11,
25
]
],
"date-time": "2022-11-25T05:51:48Z",
"timestamp": 1669355508418
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
11,
24
]
]
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
11,
24
]
],
"date-time": "2022-11-24T00:00:00Z",
"timestamp": 1669248000000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
11,
24
]
],
"date-time": "2022-11-24T00:00:00Z",
"timestamp": 1669248000000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/ptr.7640",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/ptr.7640",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/ptr.7640",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2022,
11,
24
]
]
},
"published-online": {
"date-parts": [
[
2022,
11,
24
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1089/jmf.2018.4303",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_2_1"
},
{
"DOI": "10.1136/bmjebm-2020-111336",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_3_1"
},
{
"DOI": "10.1136/bmj.m3379",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_4_1"
},
{
"DOI": "10.1016/j.hermed.2020.100404",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_5_1"
},
{
"article-title": "Effect of natural honey on human platelets and blood coagulation proteins",
"author": "Ahmed A.",
"first-page": "389",
"issue": "3",
"journal-title": "Pakistan Journal of Pharmaceutical Sciences",
"key": "e_1_2_12_6_1",
"volume": "24",
"year": "2011"
},
{
"DOI": "10.3390/molecules25215017",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_7_1"
},
{
"DOI": "10.3390/molecules26051232",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_8_1"
},
{
"DOI": "10.1038/s41598-021-91089-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_9_1"
},
{
"article-title": "Effect of Nigella sativa and bee honey on pulmonary, hepatic and renal function in Sudanese in Khartoum state",
"author": "Ameen N. M. A.",
"first-page": "6857",
"issue": "31",
"journal-title": "Journal of Medicinal Plants Research",
"key": "e_1_2_12_10_1",
"volume": "5",
"year": "2011"
},
{
"article-title": "Mechanistic similarity of immuno‐modulatory and anti‐viral effects of chloroquine and quercetin (the naturally occurring flavonoid)",
"author": "Anwar E.",
"first-page": "1",
"issue": "1",
"journal-title": "Clinical Immunology and Research",
"key": "e_1_2_12_11_1",
"volume": "4",
"year": "2020"
},
{
"DOI": "10.1186/s13063-021-05510-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_12_1"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_13_1"
},
{
"DOI": "10.5530/ijper.50.3.9",
"article-title": "Effects of Nigella sativa (Kalonji) and honey on lipid profile of hyper lipidemic smokers",
"author": "Bhatti I.",
"doi-asserted-by": "crossref",
"first-page": "376",
"issue": "3",
"journal-title": "Indian Journal of Pharmaceutical Education and Research",
"key": "e_1_2_12_14_1",
"volume": "50",
"year": "2016"
},
{
"DOI": "10.1056/NEJMoa2001282",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_15_1"
},
{
"DOI": "10.3390/molecules23092322",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_16_1"
},
{
"DOI": "10.3389/fimmu.2020.01451",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_17_1"
},
{
"DOI": "10.1016/j.phrs.2015.03.011",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_18_1"
},
{
"DOI": "10.2147/ijgm.S318720",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_19_1"
},
{
"DOI": "10.2147/ijgm.S318949",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_20_1"
},
{
"article-title": "TaibUVID nutritional supplements help rapid cure of COVID‐19 infection and rapid reversion to negative nasopharyngeal swab PCR: For better public prophylaxis and treatment of COVID‐19 pandemic",
"author": "El Sayed S. M.",
"first-page": "397",
"issue": "6",
"journal-title": "American Journal of Blood Research",
"key": "e_1_2_12_21_1",
"volume": "10",
"year": "2020"
},
{
"article-title": "Promising preventive and therapeutic effects of TaibUVID nutritional supplements for COVID‐19 pandemic: Towards better public prophylaxis and treatment (a retrospective study)",
"author": "El Sayed S. M.",
"first-page": "266",
"issue": "5",
"journal-title": "American Journal of Blood Research",
"key": "e_1_2_12_22_1",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/j.nut.2020.110948",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_23_1"
},
{
"DOI": "10.1016/j.jep.2016.06.061",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_24_1"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_25_1"
},
{
"DOI": "10.1056/NEJMoa2022926",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_26_1"
},
{
"DOI": "10.1001/jama.2021.2747",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_27_1"
},
{
"DOI": "10.7326/m20-8148",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_28_1"
},
{
"DOI": "10.1080/13880209.2021.1931353",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_29_1"
},
{
"DOI": "10.1016/j.ctim.2021.102769",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_30_1"
},
{
"DOI": "10.1016/j.curtheres.2020.100602",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_31_1"
},
{
"DOI": "10.1016/j.phymed.2020.153277",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_32_1"
},
{
"DOI": "10.1002/iub.578",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_33_1"
},
{
"DOI": "10.1093/jac/dkab093",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_34_1"
},
{
"DOI": "10.1001/jama.2020.10044",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_35_1"
},
{
"DOI": "10.1136/bmjopen-2020-039519",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_36_1"
},
{
"DOI": "10.1136/bmj.m1461",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_37_1"
},
{
"DOI": "10.1177/1178633717702869",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_38_1"
},
{
"DOI": "10.22317/jcms.v5i1.517",
"article-title": "Evaluation of Nigella sativa and honey combination for treatment of kidney stone: A randomized, placebo controlled clinical trial",
"author": "Moghimipour E.",
"doi-asserted-by": "crossref",
"first-page": "24",
"issue": "1",
"journal-title": "Journal of Contemporary Medical Sciences",
"key": "e_1_2_12_39_1",
"volume": "5",
"year": "2019"
},
{
"DOI": "10.1056/NEJMoa2023184",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_40_1"
},
{
"DOI": "10.1186/s12906-020-03170-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_41_1"
},
{
"DOI": "10.1093/advances/nmz013",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_42_1"
},
{
"DOI": "10.1136/bmj.k3651",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_43_1"
},
{
"DOI": "10.1007/s11356-021-14195-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_44_1"
},
{
"DOI": "10.1016/j.ejphar.2021.173890",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_45_1"
},
{
"DOI": "10.3831/kpi.2017.20.021",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_46_1"
},
{
"DOI": "10.1126/science.abd3255",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_47_1"
},
{
"DOI": "10.1007/s11033-014-3019-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_48_1"
},
{
"DOI": "10.1002/jlb.3covr0520-272r",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_49_1"
}
],
"reference-count": 48,
"references-count": 48,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/ptr.7640"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology"
],
"subtitle": [],
"title": "Honey and\n <scp>\n <i>Nigella sativa</i>\n </scp>\n against\n <scp>COVID</scp>\n ‐19 in Pakistan (\n <scp>HNS‐COVID‐PK</scp>\n ): A multicenter placebo‐controlled randomized clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy"
}