The effect of Nigella sativa on the risk of mortality in patients with COVID‐19: A systematic review and meta‐analysis of randomized trials
et al., Phytotherapy Research, doi:10.1002/ptr.7743, Feb 2023
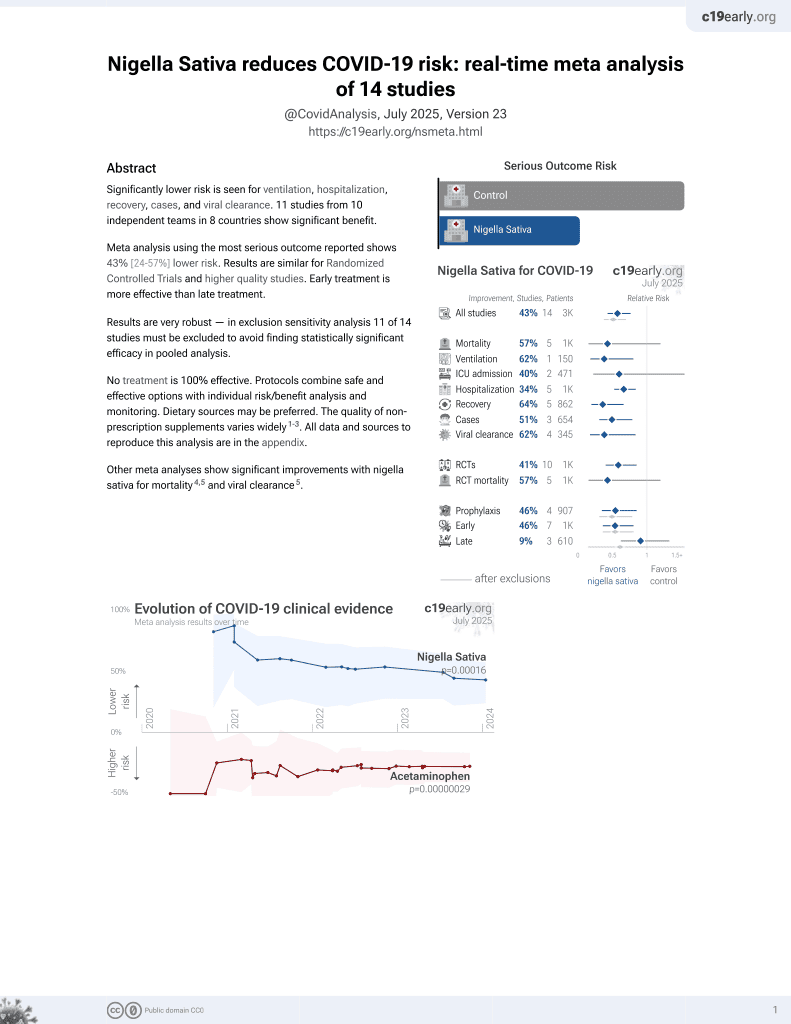
14th treatment shown to reduce risk in
January 2021, now with p = 0.00016 from 14 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
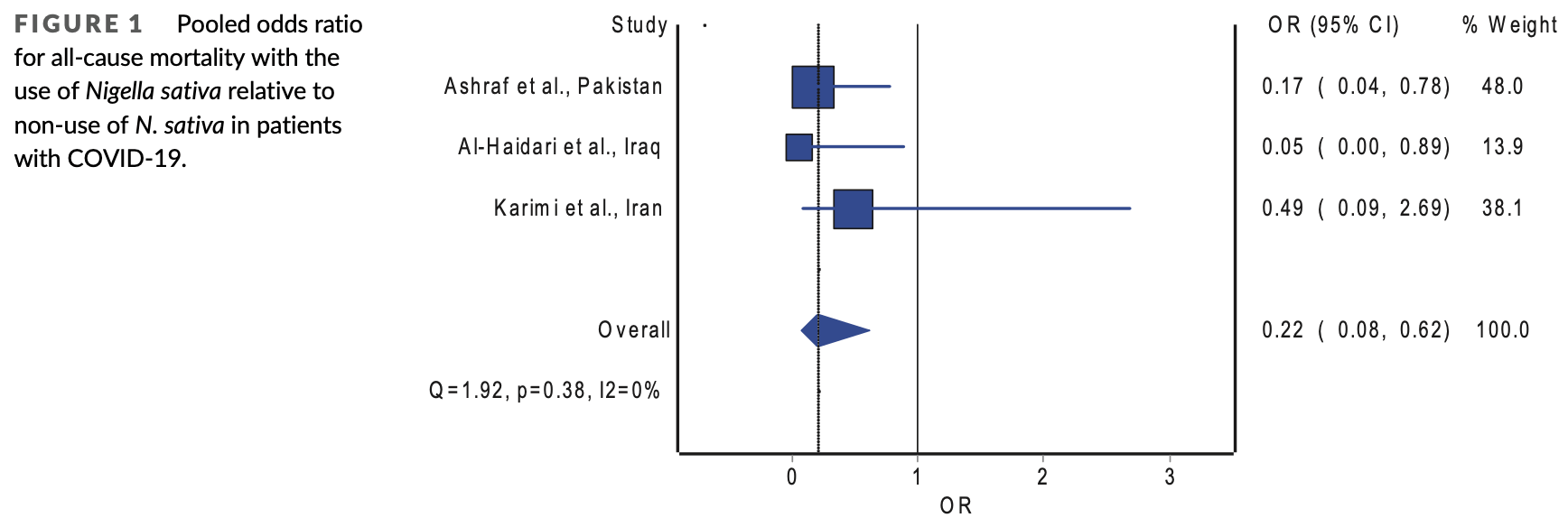
Systematic review and meta analysis of nigella sativa COVID-19 RCTs, showing significantly lower mortality with treatment.
3 meta-analyses show significant improvements with nigella sativa for mortality1-3,
progression3, and
viral clearance2.
Currently there are 14 nigella sativa for COVID-19 studies, showing 57% lower mortality [-20‑85%], 62% lower ventilation [19‑82%], 40% lower ICU admission [-61‑78%], 34% lower hospitalization [16‑47%], and 51% fewer cases [21‑69%].
|
risk of death, 78.0% lower, OR 0.22, p = 0.004, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Kow et al., The effect of Nigella sativa on the risk of mortality in patients with COVID‐19: A systematic review and meta‐analysis of randomized trials, Phytotherapy Research, doi:10.1002/ptr.7743.
Kow et al., 9 Feb 2023, peer-reviewed, 3 authors.
Contact: chiasiang_93@hotmail.com.
The effect of Nigella sativa on the risk of mortality in patients with COVID‐19: A systematic review and meta‐analysis of randomized trials
Phytotherapy Research, doi:10.1002/ptr.7743
The coronavirus disease 2019 (COVID-19) pandemic has challenged the healthcare systems given the uncontrolled spread and high mortality rate of patients with severe course of illness. In addition, anger and frustration with the pandemic are widespread due to the negative impacts of COVID-19 on social, mental, and physical well-being. Therefore, the hunt for safe and effective treatment for COVID-19 should be continued to wipe out this deadly disease. To this end, supplemental herbal remedies are being investigated as a potential treatment for COVID-19 due to their abundance of physiologically active components. Owing to its wide range of pharmacological actions, including its antiinflammatory, anti-viral, and immunomodulatory effects, Nigella sativa is specifically recommended as a possible phytomedicine for COVID-19 (Khazdair, Ghafari, & Sadeghi, 2021). It is a medicinal herb commonly referred to as "Black Cumin," and it is one of the most treasured nutrient-rich herbs in history worldwide. Even before the pandemic, N. sativa has been demonstrated to possess antiviral activities against a wide range of viruses (Koshak & Koshak, 2020) . Also, by producing several cellular mediators and immunological responses to combat infections, N. sativa has been demonstrated to possess immunostimulant effects (Gholamnezhad, Boskabady, & Hosseini, 2014; Majdalawieh & Fayyad, 2015) . In addition, by lowering proinflammatory mediators, N. sativa has also displayed antiinflammatory properties in inflammatory diseases (Bordoni et al., 2019; Hadi, Kheirouri, Alizadeh, Khabbazi, & Hosseini, 2016) . Previously in this journal, the randomized trial reported by Karimi et al. (2021) showed N. sativa accelerated the clinical recovery of patients with COVID-19. Nevertheless, the ability of this herbal remedy to reduce the risk of mortality in patients with COVID-19 may have been overlooked. Several randomized trials have been performed to investigate the effects of N. sativa on mortality reduction in patients with COVID-19. We aimed to summarize their overall mortality benefits in a systematic review and meta-analysis.
mediated immune responses (Salem, 2005) . On the other hand, antiviral effects of N. sativa against SARS-CoV-2 have been indicated by the efficient binding of the constituents of N. sativa such as α-hederin, thymohydroquinone, and thymoquinone to angiotensin-converting enzyme 2 (ACE2), the entry receptor of SARS-CoV-2, in molecular docking analyses, and thus preventing viral entry into host cells (Jakhmola Mani, Sehgal, Dogra, Saxena, & Pande Katare, 2022) . Nevertheless, the safety of N. sativa in patients with COVID-19 should be further investigated since the included trials did not report on safety outcomes. Furthermore, while no major safety concerns are reported in the literature with the consumption of N. sativa, some minor adverse effects, including nausea, bloating, and burning sensation, have been observed with the administration of N. sativa seed oil in functional dyspeptic patients, as well as a slight increase in liver enzymes (Tavakkoli, Mahdian, Razavi, & Hosseinzadeh, 2017) . Several limitations should be considered while reviewing the findings of our study. Firstly, our systematic review and metaanalysis included only three randomized trials, where some trials are with methodological flaws. Further large-scale, well-planned trials are required to confirm our findings. Nevertheless, we believe our findings should best be interpreted as an initial lead to guide efforts for more investigations of the use of N. sativa in patients with COVID-19 to..
References
Al-Haidari, Faiq, Ghareeb, Clinical trial of black seeds against COVID-19 in Kirkuk City/Iraq, Indian Journal of Forensic Medicine and Toxicology
Ashraf, Ashraf, Ashraf, Imran, Kalsoom et al., Honey and Nigella sativa against COVID-19 in Pakistan (HNS-COVID-PK): A multi-center placebo-controlled randomized clinical trial, Phytotherapy research
Bordoni, Fedeli, Nasuti, Maggi, Papa et al., Antioxidant and anti-inflammatory properties of Nigella sativa oil in human pre-adipocytes, Antioxidants
Gholamnezhad, Boskabady, Hosseini, Effect of Nigella sativa on immune response in treadmill exercised rat, BMC Complementary and Alternative Medicine
Hadi, Kheirouri, Alizadeh, Khabbazi, Hosseini, Effects of Nigella sativa oil extract on inflammatory cytokine response and oxidative stress status in patients with rheumatoid arthritis: A randomized, double-blind, placebo-controlled clinical trial, Avicenna Journal of Phytomedicine
Islam, Hossain, Sarker, Ferdous, Hannan et al., Revisiting pharmacological potentials of Nigella sativa seed: A promising option for COVID-19 prevention and cure, Phytotherapy Research
Jakhmola Mani, Sehgal, Dogra, Saxena, Pande Katare, Deciphering underlying mechanism of Sars-CoV-2 infection in humans and revealing the therapeutic potential of bioactive constituents from Nigella sativa to combat COVID19: In-silico study, Journal of Biomolecular Structure & Dynamics
Karimi, Zarei, Soleymani, Jamalimoghadamsiahkali, Asadi et al., Efficacy of Persian medicine herbal formulations (capsules and decoction) compared to standard care in patients with COVID-19, a multicenter open-labeled, randomized, controlled clinical trial, Phytotherapy Research
Khazdair, Ghafari, Sadeghi, Possible therapeutic effects of Nigella sativa and its thymoquinone on COVID-19, Pharmaceutical Biology
Koshak, Koshak, Nigella sativa L as a potential phytotherapy for coronavirus disease 2019: A mini review of in silico studies
Majdalawieh, Fayyad, Immunomodulatory and anti-inflammatory action of Nigella sativa and thymoquinone: A comprehensive review, International Immunopharmacology
Page, Mckenzie, Bossuyt, Boutron, Hoffmann et al., The PRISMA 2020 statement: An updated guideline for reporting systematic reviews, BMJ
Salem, Immunomodulatory and therapeutic properties of the Nigella sativa L. seed, International Immunopharmacology
Sterne, Savovi C, Page, Elbers, Blencowe et al., RoB 2: A revised tool for assessing risk of bias in randomised trials, BMJ
Tavakkoli, Mahdian, Razavi, Hosseinzadeh, Review on clinical trials of black seed (Nigella sativa) and its active constituent, thymoquinone, Journal of Pharmacopuncture
DOI record:
{
"DOI": "10.1002/ptr.7743",
"ISSN": [
"0951-418X",
"1099-1573"
],
"URL": "http://dx.doi.org/10.1002/ptr.7743",
"alternative-id": [
"10.1002/ptr.7743"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-07-05"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-12-18"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2023-02-09"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-8186-2926",
"affiliation": [
{
"name": "School of Postgraduate Studies International Medical University Kuala Lumpur Malaysia"
},
{
"name": "School of Pharmacy Monash University Malaysia Bandar Sunway Malaysia"
}
],
"authenticated-orcid": false,
"family": "Kow",
"given": "Chia Siang",
"sequence": "first"
},
{
"affiliation": [
{
"name": "School of Pharmacy Monash University Malaysia Bandar Sunway Malaysia"
}
],
"family": "Ramachandram",
"given": "Dinesh Sangarran",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Applied Sciences University of Huddersfield Huddersfield UK"
},
{
"name": "School of Biomedical Sciences and Pharmacy University of Newcastle Callaghan New South Wales Australia"
}
],
"family": "Hasan",
"given": "Syed Shahzad",
"sequence": "additional"
}
],
"container-title": "Phytotherapy Research",
"container-title-short": "Phytotherapy Research",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2023,
2,
9
]
],
"date-time": "2023-02-09T12:20:25Z",
"timestamp": 1675945225000
},
"deposited": {
"date-parts": [
[
2023,
6,
4
]
],
"date-time": "2023-06-04T13:16:39Z",
"timestamp": 1685884599000
},
"indexed": {
"date-parts": [
[
2023,
6,
5
]
],
"date-time": "2023-06-05T08:01:27Z",
"timestamp": 1685952087862
},
"is-referenced-by-count": 1,
"issued": {
"date-parts": [
[
2023,
2,
9
]
]
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
2,
9
]
],
"date-time": "2023-02-09T00:00:00Z",
"timestamp": 1675900800000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/ptr.7743",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/ptr.7743",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/ptr.7743",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2023,
2,
9
]
]
},
"published-online": {
"date-parts": [
[
2023,
2,
9
]
]
},
"publisher": "Wiley",
"reference": [
{
"article-title": "Clinical trial of black seeds against COVID‐19 in Kirkuk City/Iraq",
"author": "Al‐Haidari K. A. A.",
"first-page": "3393",
"issue": "3",
"journal-title": "Indian Journal of Forensic Medicine and Toxicology",
"key": "e_1_2_1_7_2_1",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1002/ptr.7640",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_7_3_1"
},
{
"DOI": "10.3390/antiox8020051",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_7_4_1"
},
{
"DOI": "10.1186/1472-6882-14-437",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_7_5_1"
},
{
"article-title": "Effects of Nigella sativa oil extract on inflammatory cytokine response and oxidative stress status in patients with rheumatoid arthritis: A randomized, double‐blind, placebo‐controlled clinical trial",
"author": "Hadi V.",
"first-page": "34",
"issue": "1",
"journal-title": "Avicenna Journal of Phytomedicine",
"key": "e_1_2_1_7_6_1",
"volume": "6",
"year": "2016"
},
{
"DOI": "10.1002/ptr.6895",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_7_7_1"
},
{
"DOI": "10.1080/07391102.2020.1839560",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_7_8_1"
},
{
"DOI": "10.1002/ptr.7277",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_7_9_1"
},
{
"DOI": "10.1080/13880209.2021.1931353",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_7_10_1"
},
{
"DOI": "10.1016/j.curtheres.2020.100602",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_7_11_1"
},
{
"DOI": "10.1016/j.intimp.2015.06.023",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_7_12_1"
},
{
"DOI": "10.1136/bmj.n71",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_7_13_1"
},
{
"DOI": "10.1016/j.intimp.2005.06.008",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_7_14_1"
},
{
"DOI": "10.1136/bmj.l4898",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_7_15_1"
},
{
"DOI": "10.3831/KPI.2017.20.021",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_7_16_1"
}
],
"reference-count": 15,
"references-count": 15,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/ptr.7743"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology"
],
"subtitle": [],
"title": "The effect of <i>Nigella sativa</i> on the risk of mortality in patients with <scp>COVID</scp>‐19: A systematic review and meta‐analysis of randomized trials",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy"
}