Clinical and laboratory characteristics of patients hospitalized with severe COVID-19 in New Orleans, August 2020 to September 2021
et al., Scientific Reports, doi:10.1038/s41598-024-57306-5, Mar 2024
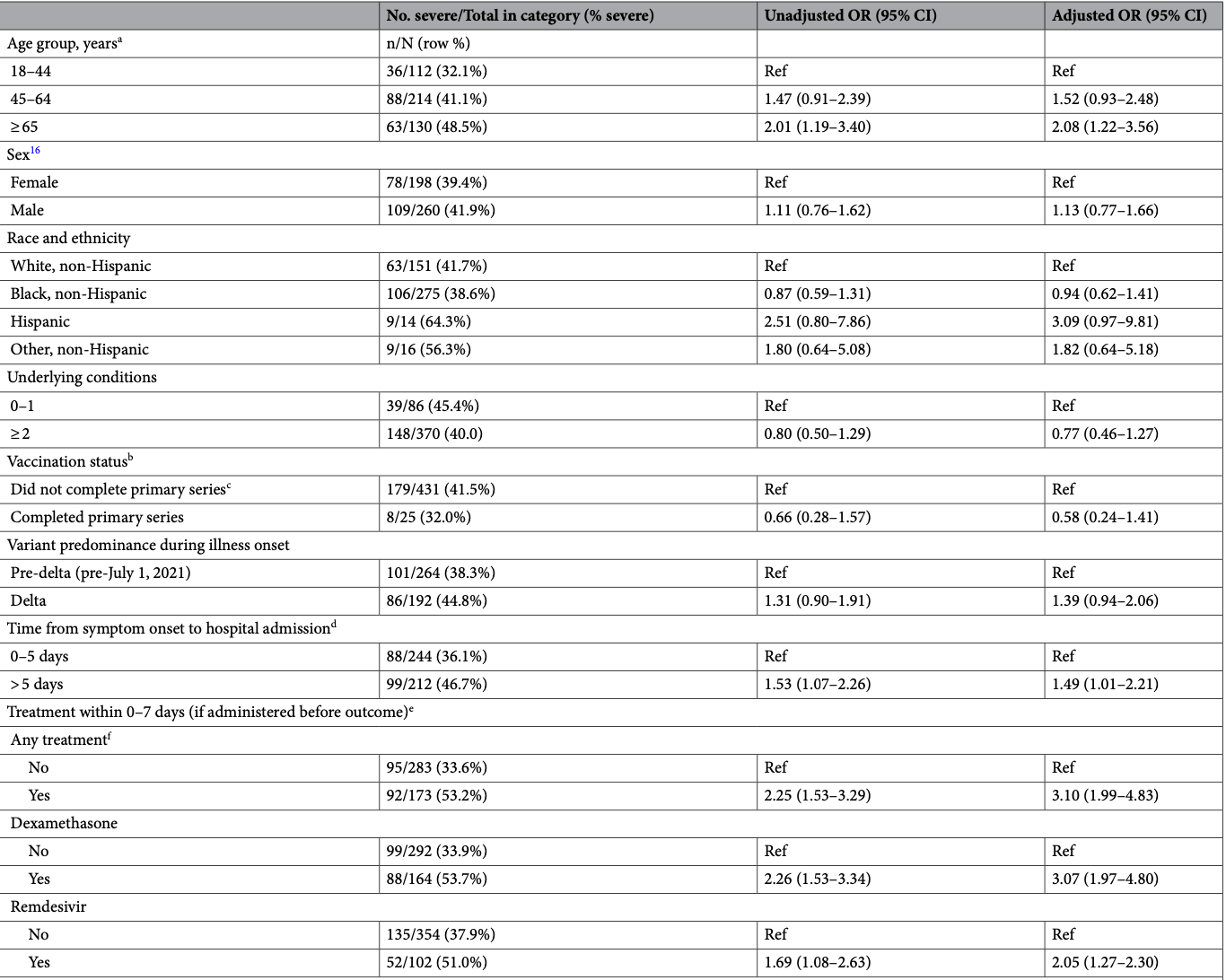
Retrospective 456 hospitalized patients in the USA showing an association between remdesivir treatment and increased COVID-19 severity in multivariable analysis, for remdesivir treatment within 7 days and when administered before meeting the severe case definition. Authors suggest this is due to remdesivir being preferentially used for more severe cases, citing Bhimraj et al., however that paper is from April 2020 before widespread use of remdesivir. During the period of the current study remdesivir was more widely recommended. However, there could still be significant residual confounding after adjustments.
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments15.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This study is excluded in the after exclusion results of meta-analysis:
substantial unadjusted confounding by indication likely.
|
risk of severe case, 46.4% higher, RR 1.46, p < 0.001, treatment 52 of 102 (51.0%), control 135 of 354 (38.1%), adjusted per study, odds ratio converted to relative risk, multivariable.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
2.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
3.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
4.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
5.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
6.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
7.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
8.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
9.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
10.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
11.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
12.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
13.
Mohammed et al., Bradycardia associated with remdesivir treatment in coronavirus disease 2019 patients: A propensity score-matched analysis, Medicine, doi:10.1097/MD.0000000000044501.
Drouin et al., 19 Mar 2024, retrospective, USA, peer-reviewed, median age 56.0, 13 authors, study period August 2020 - September 2021.
Contact: dfusco@tulane.edu.
Clinical and laboratory characteristics of patients hospitalized with severe COVID-19 in New Orleans, August 2020 to September 2021
Scientific Reports, doi:10.1038/s41598-024-57306-5
Louisiana experienced high morbidity and mortality from COVID-19. To assess possible explanatory factors, we conducted a cohort study (ClinSeqSer) of patients hospitalized with COVID-19 in New Orleans during August 2020-September 2021. Following enrollment, we reviewed medical charts, and performed SARS-CoV-2 RT-PCR testing on nasal and saliva specimens. We used multivariable logistic regression to assess associations between patient characteristics and severe illness, defined as ≥ 6 L/min oxygen or intubation. Among 456 patients, median age was 56 years, 277 (60.5%) were Black non-Hispanic, 436 (95.2%) had underlying health conditions, and 358 were unvaccinated (92.0% of 389 verified). Overall, 187 patients (40.1%) had severe illness; 60 (13.1%) died during admission. In multivariable models, severe illness was associated with age ≥ 65 years (OR 2.08, 95% CI 1.22-3.56), hospitalization > 5 days after illness onset (OR 1.49, 95% CI 1.01-2.21), and SARS CoV-2 cycle threshold (Ct) result of < 32 in saliva (OR 4.79, 95% CI 1.22-18.77). Among patients who were predominantly Black non-Hispanic, unvaccinated and with underlying health conditions, approximately 1 in 3 patients had severe COVID-19. Older age and delayed time to admission might have contributed to high case-severity. An association between case-severity and low Ct value in saliva warrants further investigation. Keywords COVID-19,
Ethical approval This study was reviewed and approved by the Tulane University School of Medicine Institutional Review Board, and was performed in accordance with relevant guidelines (see 45 C.F.R. part 46; 21 C.F.R. part 56). Informed consent was obtained from all participants and/or their legal guardians.
Disclaimer The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Author contributions The analysis was planned by A.D, D.F., I.P., and C.M. with input from other coauthors. Data preparation and analysis was conducted by D.F., M.M., and J.J.G. Data interpretation was conducted by D.F., C.M., and I.P. and manuscript writing was completed by D.F. and I.P. with critical input from all authors. D.F. had full access to all the data in the study and takes responsibility for the integrity of the data. All authors certify that they meet authorship criteria.
Competing interests DF has served on an Advisory Board for Gilead Sciences and for AXCELLA, and as site PI for clinical trials sponsored by Gilead Sciences, Regeneron, MetroBiotech LLC, and the NIH (DMID COVAIL). AD has served as co site-PI on clinical trials sponsored by Gilead Sciences, Regeneron, MetroBiotech LLC, and the NIH (DMID COVAIL). Other authors declare that they have no conflict of interest.
References
Bambra, Pandemic inequalities: Emerging infectious diseases and health equity, Int. J. Equity Health
Bhimraj, Infectious diseases society of america guidelines on the treatment and management of patients with coronavirus disease, COVID
Bobrovitz, Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: A systematic review and meta-regression, Lancet Infect. Dis
Carabelli, SARS-CoV-2 variant biology: Immune escape, transmission and fitness, Nat. Rev. Microbiol
Cdc, Centers for disease control and prevention
Choudhuri, SARS-CoV-2 PCR cycle threshold at hospital admission associated with patient mortality, PLOS ONE
Congrave-Wilson, Change in saliva RT-PCR sensitivity over the course of SARS-CoV-2 infection, JAMA
Davis, Long COVID: Major findings, mechanisms and recommendations, Nat. Rev. Microbiol
De La Calle, Impact of viral load at admission on the development of respiratory failure in hospitalized patients with SARS-CoV-2 infection, Eur. J. Clin. Microbiol. Infect. Dis
Gao, Risk factors for severe and critically ill COVID-19 patients: A review, Allergy
Garibaldi, Patient trajectories among persons hospitalized for COVID-19: A cohort study, Ann. Intern. Med
Gold, Characteristics and clinical outcomes of adult patients hospitalized with COVID-19 -Georgia, March 2020, MMWR Morb. Mortal Wkly Rep
Goldberg, A real-life setting evaluation of the effect of remdesivir on viral load in COVID-19 patients admitted to a large tertiary centre in Israel, Clin. Microbiol. Infect
Gottlieb, Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: A randomized clinical trial, JAMA
Griffith, Collider bias undermines our understanding of COVID-19 disease risk and severity, Nat. Commun
Gupta, Extrapulmonary manifestations of COVID-19, Nat. Med
Haldane, Health systems resilience in managing the COVID-19 pandemic: Lessons from 28 countries, Nat. Med
Huang, SARS-CoV-2 infection of the oral cavity and saliva, Nat. Med
Idsa, IDSA COVID treatment, doi:10.1093/cid/ciac724/6692369
Jones, Estimated US infection-and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations, July 2020-May 2021, JAMA
Julio, Saliva viral load is a dynamic unifying correlate of COVID-19 severity and mortality, Scientific Reports, doi:10.1038/s41598-024-57306-5www.nature.com/scientificreports/
Killingley, Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults, Nat. Med
Koelle, The changing epidemiology of SARS-CoV-2, Science
Magleby, Impact of severe acute respiratory syndrome coronavirus 2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019, Clin. Infect. Dis
Merad, The immunology and immunopathology of COVID-19, Science
Orner, Comparison of SARS-CoV-2 IgM and IgG seroconversion profiles among hospitalized patients in two US cities, Diagn. Microbiol. Infect. Dis
Price-Haywood, Hospitalization and mortality among black patients and white patients with Covid-19, N. Engl. J. Med
Procop, A direct comparison of enhanced saliva to nasopharyngeal swab for the detection of SARS-CoV-2 in symptomatic patients, J. Clin. Microbiol, doi:10.1128/jcm.01946-20
Rico-Caballero, Impact of SARS-CoV-2 viral load and duration of symptoms before hospital admission on the mortality of hospitalized COVID-19 patients, Infection
Russell, Lone, Baillie, Comorbidities, multimorbidity and COVID-19, Nat. Med
Scobie, Monitoring incidence of COVID-19 cases, hospitalizations, and deaths, by vaccination status -13 U.S. jurisdictions, MMWR Morb. Mortal Wkly. Rep
Tanner, SARS-CoV-2 viral load at presentation to hospital is independently associated with the risk of death, J. Infect
Taylor, Severity of disease among adults hospitalized with laboratory-confirmed COVID-19 before and during the period of SARS-CoV-2 B.1.617.2 (Delta) predominance -COVID-NET, 14 States, MMWR Morb. Mortal Wkly. Rep
To, Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study, Lancet Infect. Dis
Victora, The role of conceptual frameworks in epidemiological analysis: A hierarchical approach, Int. J. Epidemiol
Walsh, SARS-CoV-2 detection, viral load and infectivity over the course of an infection, J. Infect
Williamson, Factors associated with COVID-19-related death using OpenSAFELY, Nature
Wyllie, Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2, N. Engl. J. Med
Zhou, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study, Lancet
DOI record:
{
"DOI": "10.1038/s41598-024-57306-5",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-024-57306-5",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Louisiana experienced high morbidity and mortality from COVID-19. To assess possible explanatory factors, we conducted a cohort study (ClinSeqSer) of patients hospitalized with COVID-19 in New Orleans during August 2020–September 2021. Following enrollment, we reviewed medical charts, and performed SARS-CoV-2 RT-PCR testing on nasal and saliva specimens. We used multivariable logistic regression to assess associations between patient characteristics and severe illness, defined as ≥ 6 L/min oxygen or intubation. Among 456 patients, median age was 56 years, 277 (60.5%) were Black non-Hispanic, 436 (95.2%) had underlying health conditions, and 358 were unvaccinated (92.0% of 389 verified). Overall, 187 patients (40.1%) had severe illness; 60 (13.1%) died during admission. In multivariable models, severe illness was associated with age ≥ 65 years (OR 2.08, 95% CI 1.22–3.56), hospitalization > 5 days after illness onset (OR 1.49, 95% CI 1.01–2.21), and SARS CoV-2 cycle threshold (Ct) result of < 32 in saliva (OR 4.79, 95% CI 1.22–18.77). Among patients who were predominantly Black non-Hispanic, unvaccinated and with underlying health conditions, approximately 1 in 3 patients had severe COVID-19. Older age and delayed time to admission might have contributed to high case-severity. An association between case-severity and low Ct value in saliva warrants further investigation.</jats:p>",
"alternative-id": [
"57306"
],
"article-number": "6539",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "18 December 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "17 March 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "19 March 2024"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "DF has served on an Advisory Board for Gilead Sciences and for AXCELLA, and as site PI for clinical trials sponsored by Gilead Sciences, Regeneron, MetroBiotech LLC, and the NIH (DMID COVAIL). AD has served as co site-PI on clinical trials sponsored by Gilead Sciences, Regeneron, MetroBiotech LLC, and the NIH (DMID COVAIL). Other authors declare that they have no conflict of interest."
}
],
"author": [
{
"affiliation": [],
"family": "Drouin",
"given": "Arnaud",
"sequence": "first"
},
{
"affiliation": [],
"family": "Plumb",
"given": "Ian D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McCullough",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "James Gist",
"given": "Jade",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Sharon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Theberge",
"given": "Marc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Katz",
"given": "Joshua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moreida",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Flaherty",
"given": "Shelby",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chatwani",
"given": "Bhoomija",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Briggs Hagen",
"given": "Melissa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Midgley",
"given": "Claire M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fusco",
"given": "Dahlene",
"sequence": "additional"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
3,
19
]
],
"date-time": "2024-03-19T06:50:29Z",
"timestamp": 1710831029000
},
"deposited": {
"date-parts": [
[
2024,
3,
19
]
],
"date-time": "2024-03-19T06:54:35Z",
"timestamp": 1710831275000
},
"funder": [
{
"DOI": "10.13039/100000030",
"award": [
"U01CK000480"
],
"doi-asserted-by": "publisher",
"name": "Centers for Disease Control and Prevention"
}
],
"indexed": {
"date-parts": [
[
2024,
3,
26
]
],
"date-time": "2024-03-26T02:28:13Z",
"timestamp": 1711420093774
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
3,
19
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
3,
19
]
],
"date-time": "2024-03-19T00:00:00Z",
"timestamp": 1710806400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
3,
19
]
],
"date-time": "2024-03-19T00:00:00Z",
"timestamp": 1710806400000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-024-57306-5.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-024-57306-5",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-024-57306-5.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2024,
3,
19
]
]
},
"published-online": {
"date-parts": [
[
2024,
3,
19
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1038/s41579-022-00846-2",
"author": "HE Davis",
"doi-asserted-by": "publisher",
"first-page": "133",
"issue": "3",
"journal-title": "Nat. Rev. Microbiol.",
"key": "57306_CR1",
"unstructured": "Davis, H. E. et al. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 21(3), 133–146 (2023).",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1038/s41591-020-0968-3",
"author": "A Gupta",
"doi-asserted-by": "publisher",
"first-page": "1017",
"issue": "7",
"journal-title": "Nat. Med.",
"key": "57306_CR2",
"unstructured": "Gupta, A. et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 26(7), 1017–1032 (2020).",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1126/science.abm4915",
"author": "K Koelle",
"doi-asserted-by": "publisher",
"first-page": "1116",
"issue": "6585",
"journal-title": "Science",
"key": "57306_CR3",
"unstructured": "Koelle, K. et al. The changing epidemiology of SARS-CoV-2. Science 375(6585), 1116–1121 (2022).",
"volume": "375",
"year": "2022"
},
{
"key": "57306_CR4",
"unstructured": "CDC COVID 19 mortality final. (2023); Available from: https://www.cdc.gov/nchs/pressroom/sosmap/covid19_mortality_final/COVID19.htm"
},
{
"DOI": "10.1056/NEJMsa2011686",
"author": "EG Price-Haywood",
"doi-asserted-by": "publisher",
"first-page": "2534",
"issue": "26",
"journal-title": "N. Engl. J. Med.",
"key": "57306_CR5",
"unstructured": "Price-Haywood, E. G. et al. Hospitalization and mortality among black patients and white patients with Covid-19. N. Engl. J. Med. 382(26), 2534–2543 (2020).",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1186/s12939-021-01611-2",
"author": "C Bambra",
"doi-asserted-by": "publisher",
"first-page": "6",
"issue": "1",
"journal-title": "Int. J. Equity Health",
"key": "57306_CR6",
"unstructured": "Bambra, C. Pandemic inequalities: Emerging infectious diseases and health equity. Int. J. Equity Health 21(1), 6 (2022).",
"volume": "21",
"year": "2022"
},
{
"DOI": "10.1038/s41586-020-2521-4",
"author": "EJ Williamson",
"doi-asserted-by": "publisher",
"first-page": "430",
"issue": "7821",
"journal-title": "Nature",
"key": "57306_CR7",
"unstructured": "Williamson, E. J. et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 584(7821), 430–436 (2020).",
"volume": "584",
"year": "2020"
},
{
"DOI": "10.1111/all.14657",
"author": "YD Gao",
"doi-asserted-by": "publisher",
"first-page": "428",
"issue": "2",
"journal-title": "Allergy",
"key": "57306_CR8",
"unstructured": "Gao, Y. D. et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 76(2), 428–455 (2021).",
"volume": "76",
"year": "2021"
},
{
"DOI": "10.1126/science.abm8108",
"author": "M Merad",
"doi-asserted-by": "publisher",
"first-page": "1122",
"issue": "6585",
"journal-title": "Science",
"key": "57306_CR9",
"unstructured": "Merad, M. et al. The immunology and immunopathology of COVID-19. Science 375(6585), 1122–1127 (2022).",
"volume": "375",
"year": "2022"
},
{
"key": "57306_CR10",
"unstructured": "CDC Geographic Distribution of Diabetes in US. (2023); Available from: https://www.cdc.gov/diabetes/library/reports/reportcard/national-state-diabetes-trends.html"
},
{
"key": "57306_CR11",
"unstructured": "CDC Obesity Data Prevalence. (2023); Available from: https://www.cdc.gov/obesity/data/prevalence-maps.html"
},
{
"DOI": "10.1038/s41591-022-02156-9",
"author": "CD Russell",
"doi-asserted-by": "publisher",
"first-page": "334",
"issue": "2",
"journal-title": "Nat. Med.",
"key": "57306_CR12",
"unstructured": "Russell, C. D., Lone, N. I. & Baillie, J. K. Comorbidities, multimorbidity and COVID-19. Nat. Med. 29(2), 334–343 (2023).",
"volume": "29",
"year": "2023"
},
{
"author": "AM Carabelli",
"first-page": "162",
"issue": "3",
"journal-title": "Nat. Rev. Microbiol.",
"key": "57306_CR13",
"unstructured": "Carabelli, A. M. et al. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 21(3), 162–177 (2023).",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1016/j.jinf.2020.06.067",
"author": "KA Walsh",
"doi-asserted-by": "publisher",
"first-page": "357",
"issue": "3",
"journal-title": "J. Infect.",
"key": "57306_CR14",
"unstructured": "Walsh, K. A. et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J. Infect. 81(3), 357–371 (2020).",
"volume": "81",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0244777",
"author": "J Choudhuri",
"doi-asserted-by": "publisher",
"first-page": "e0244777",
"issue": "12",
"journal-title": "PLOS ONE",
"key": "57306_CR15",
"unstructured": "Choudhuri, J. et al. SARS-CoV-2 PCR cycle threshold at hospital admission associated with patient mortality. PLOS ONE 15(12), e0244777 (2021).",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1007/s10096-020-04150-w",
"author": "C de la Calle",
"doi-asserted-by": "publisher",
"first-page": "1209",
"issue": "6",
"journal-title": "Eur. J. Clin. Microbiol. Infect. Dis.",
"key": "57306_CR16",
"unstructured": "de la Calle, C. et al. Impact of viral load at admission on the development of respiratory failure in hospitalized patients with SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 40(6), 1209–1216 (2021).",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa851",
"author": "R Magleby",
"doi-asserted-by": "publisher",
"first-page": "e4197",
"issue": "11",
"journal-title": "Clin. Infect. Dis.",
"key": "57306_CR17",
"unstructured": "Magleby, R. et al. Impact of severe acute respiratory syndrome coronavirus 2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clin. Infect. Dis. 73(11), e4197–e4205 (2021).",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1007/s15010-022-01833-8",
"author": "V Rico-Caballero",
"doi-asserted-by": "publisher",
"first-page": "1321",
"issue": "5",
"journal-title": "Infection",
"key": "57306_CR18",
"unstructured": "Rico-Caballero, V. et al. Impact of SARS-CoV-2 viral load and duration of symptoms before hospital admission on the mortality of hospitalized COVID-19 patients. Infection 50(5), 1321–1328 (2022).",
"volume": "50",
"year": "2022"
},
{
"DOI": "10.1016/j.jinf.2021.08.003",
"author": "AR Tanner",
"doi-asserted-by": "publisher",
"first-page": "458",
"issue": "4",
"journal-title": "J. Infect.",
"key": "57306_CR19",
"unstructured": "Tanner, A. R. et al. SARS-CoV-2 viral load at presentation to hospital is independently associated with the risk of death. J. Infect. 83(4), 458–466 (2021).",
"volume": "83",
"year": "2021"
},
{
"DOI": "10.1038/s41467-020-19478-2",
"author": "GJ Griffith",
"doi-asserted-by": "publisher",
"first-page": "5749",
"issue": "1",
"journal-title": "Nat. Commun.",
"key": "57306_CR20",
"unstructured": "Griffith, G. J. et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat. Commun. 11(1), 5749 (2020).",
"volume": "11",
"year": "2020"
},
{
"key": "57306_CR21",
"unstructured": "New Orleans US Census Data. (2023); Available from: https://www.census.gov/quickfacts/fact/table/neworleanscitylouisiana/PST045222"
},
{
"DOI": "10.15585/mmwr.mm6918e1",
"author": "JAW Gold",
"doi-asserted-by": "publisher",
"first-page": "545",
"issue": "18",
"journal-title": "MMWR Morb. Mortal Wkly Rep.",
"key": "57306_CR22",
"unstructured": "Gold, J. A. W. et al. Characteristics and clinical outcomes of adult patients hospitalized with COVID-19 - Georgia, March 2020. MMWR Morb. Mortal Wkly Rep. 69(18), 545–550 (2020).",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"author": "F Zhou",
"doi-asserted-by": "publisher",
"first-page": "1054",
"issue": "10229",
"journal-title": "Lancet",
"key": "57306_CR23",
"unstructured": "Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395(10229), 1054–1062 (2020).",
"volume": "395",
"year": "2020"
},
{
"key": "57306_CR24",
"unstructured": "Bhimraj, A., et al., Infectious diseases society of america guidelines on the treatment and management of patients with coronavirus disease 2019 (COVID-19), ciac724 (Clinical Infectious Diseases, 2022)."
},
{
"DOI": "10.15585/mmwr.mm7037e1",
"author": "HM Scobie",
"doi-asserted-by": "publisher",
"first-page": "1284",
"issue": "37",
"journal-title": "MMWR Morb. Mortal Wkly. Rep.",
"key": "57306_CR25",
"unstructured": "Scobie, H. M. et al. Monitoring incidence of COVID-19 cases, hospitalizations, and deaths, by vaccination status - 13 U.S. jurisdictions, April 4–July 17, 2021. MMWR Morb. Mortal Wkly. Rep. 70(37), 1284–1290 (2021).",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.15161",
"author": "JM Jones",
"doi-asserted-by": "publisher",
"first-page": "1400",
"issue": "14",
"journal-title": "JAMA",
"key": "57306_CR26",
"unstructured": "Jones, J. M. et al. Estimated US infection- and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations, July 2020–May 2021. JAMA 326(14), 1400–1409 (2021).",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1016/S1473-3099(22)00801-5",
"author": "N Bobrovitz",
"doi-asserted-by": "publisher",
"first-page": "556",
"issue": "5",
"journal-title": "Lancet Infect. Dis.",
"key": "57306_CR27",
"unstructured": "Bobrovitz, N. et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: A systematic review and meta-regression. Lancet Infect. Dis. 23(5), 556–567 (2023).",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1016/j.diagmicrobio.2020.115300",
"author": "EP Orner",
"doi-asserted-by": "publisher",
"first-page": "115300",
"issue": "4",
"journal-title": "Diagn. Microbiol. Infect. Dis.",
"key": "57306_CR28",
"unstructured": "Orner, E. P. et al. Comparison of SARS-CoV-2 IgM and IgG seroconversion profiles among hospitalized patients in two US cities. Diagn. Microbiol. Infect. Dis. 99(4), 115300 (2021).",
"volume": "99",
"year": "2021"
},
{
"DOI": "10.1038/s41591-022-01780-9",
"author": "B Killingley",
"doi-asserted-by": "publisher",
"first-page": "1031",
"issue": "5",
"journal-title": "Nat. Med.",
"key": "57306_CR29",
"unstructured": "Killingley, B. et al. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nat. Med. 28(5), 1031–1041 (2022).",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(20)30196-1",
"author": "KK-W To",
"doi-asserted-by": "publisher",
"first-page": "565",
"issue": "5",
"journal-title": "Lancet Infect. Dis.",
"key": "57306_CR30",
"unstructured": "To, K.K.-W. et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 20(5), 565–574 (2020).",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1038/s41591-021-01296-8",
"author": "N Huang",
"doi-asserted-by": "publisher",
"first-page": "892",
"issue": "5",
"journal-title": "Nat. Med.",
"key": "57306_CR31",
"unstructured": "Huang, N. et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat. Med. 27(5), 892–903 (2021).",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.13967",
"author": "Z Congrave-Wilson",
"doi-asserted-by": "publisher",
"first-page": "1065",
"issue": "11",
"journal-title": "JAMA",
"key": "57306_CR32",
"unstructured": "Congrave-Wilson, Z. et al. Change in saliva RT-PCR sensitivity over the course of SARS-CoV-2 infection. JAMA 326(11), 1065–1067 (2021).",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1128/jcm.01946-20",
"author": "GW Procop",
"doi-asserted-by": "publisher",
"journal-title": "J. Clin. Microbiol.",
"key": "57306_CR33",
"unstructured": "Procop, G. W. et al. A direct comparison of enhanced saliva to nasopharyngeal swab for the detection of SARS-CoV-2 in symptomatic patients. J. Clin. Microbiol. https://doi.org/10.1128/jcm.01946-20 (2020).",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2016359",
"author": "AL Wyllie",
"doi-asserted-by": "publisher",
"first-page": "1283",
"issue": "13",
"journal-title": "N. Engl. J. Med.",
"key": "57306_CR34",
"unstructured": "Wyllie, A. L. et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N. Engl. J. Med. 383(13), 1283–1286 (2020).",
"volume": "383",
"year": "2020"
},
{
"key": "57306_CR35",
"unstructured": "Julio, S. et al. Saliva viral load is a dynamic unifying correlate of COVID-19 severity and mortality. medRxiv 2021.01.04.21249236 (2021)."
},
{
"DOI": "10.1016/j.cmi.2021.02.029",
"author": "E Goldberg",
"doi-asserted-by": "publisher",
"first-page": "917.e1",
"issue": "6",
"journal-title": "Clin. Microbiol. Infect.",
"key": "57306_CR36",
"unstructured": "Goldberg, E. et al. A real-life setting evaluation of the effect of remdesivir on viral load in COVID-19 patients admitted to a large tertiary centre in Israel. Clin. Microbiol. Infect. 27(6), 917.e1-917.e4 (2021).",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.0202",
"author": "RL Gottlieb",
"doi-asserted-by": "publisher",
"first-page": "632",
"issue": "7",
"journal-title": "JAMA",
"key": "57306_CR37",
"unstructured": "Gottlieb, R. L. et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: A randomized clinical trial. JAMA 325(7), 632–644 (2021).",
"volume": "325",
"year": "2021"
},
{
"key": "57306_CR38",
"unstructured": "NIH. NIH COVID treatment guidelines. 2024 02/07/2024]; Available from: https://www.covid19treatmentguidelines.nih.gov/"
},
{
"DOI": "10.1093/cid/ciac724/6692369",
"doi-asserted-by": "publisher",
"key": "57306_CR39",
"unstructured": "IDSA. IDSA COVID treatment guidelines. 2024 02/07/2024]; Available from: https://doi.org/10.1093/cid/ciac724/6692369."
},
{
"key": "57306_CR40",
"unstructured": "CDC. COVID data tracker. Centers for disease control and prevention 2020 2020/03/28/; Available from: https://covid.cdc.gov/covid-data-tracker."
},
{
"DOI": "10.1038/s41591-021-01381-y",
"author": "V Haldane",
"doi-asserted-by": "publisher",
"first-page": "964",
"issue": "6",
"journal-title": "Nat. Med.",
"key": "57306_CR41",
"unstructured": "Haldane, V. et al. Health systems resilience in managing the COVID-19 pandemic: Lessons from 28 countries. Nat. Med. 27(6), 964–980 (2021).",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.7326/M20-3905",
"author": "BT Garibaldi",
"doi-asserted-by": "publisher",
"first-page": "33",
"issue": "1",
"journal-title": "Ann. Intern. Med.",
"key": "57306_CR42",
"unstructured": "Garibaldi, B. T. et al. Patient trajectories among persons hospitalized for COVID-19: A cohort study. Ann. Intern. Med. 174(1), 33–41 (2021).",
"volume": "174",
"year": "2021"
},
{
"DOI": "10.15585/mmwr.mm7043e1",
"author": "CA Taylor",
"doi-asserted-by": "publisher",
"first-page": "1513",
"issue": "43",
"journal-title": "MMWR Morb. Mortal Wkly. Rep.",
"key": "57306_CR43",
"unstructured": "Taylor, C. A. et al. Severity of disease among adults hospitalized with laboratory-confirmed COVID-19 before and during the period of SARS-CoV-2 B.1.617.2 (Delta) predominance - COVID-NET, 14 States, January–August 2021. MMWR Morb. Mortal Wkly. Rep. 70(43), 1513–1519 (2021).",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.1093/ije/26.1.224",
"author": "CG Victora",
"doi-asserted-by": "publisher",
"first-page": "224",
"issue": "1",
"journal-title": "Int. J. Epidemiol.",
"key": "57306_CR44",
"unstructured": "Victora, C. G. et al. The role of conceptual frameworks in epidemiological analysis: A hierarchical approach. Int. J. Epidemiol. 26(1), 224–227 (1997).",
"volume": "26",
"year": "1997"
}
],
"reference-count": 44,
"references-count": 44,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.21203/rs.3.rs-3773246/v1",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-024-57306-5"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "Clinical and laboratory characteristics of patients hospitalized with severe COVID-19 in New Orleans, August 2020 to September 2021",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "14"
}