Effects of Hydroxychloroquine Plus Favipiravir Treatment on the Clinical Course and Biomarkers in Hospitalized COVID-19 Patients with Pneumonia
et al., Acta Clinica Croatica, doi:10.20471/acc.2022.61.03.05, Dec 2022
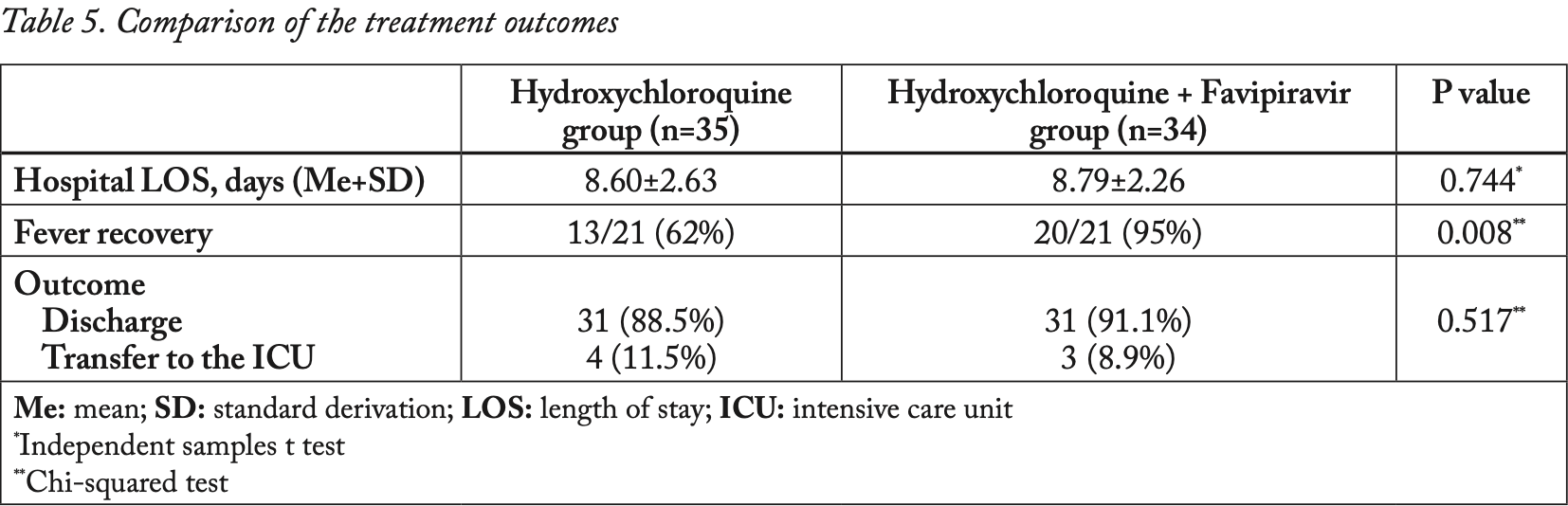
Retrospective 69 COVID-19 patients in Turkey, showing improved fever recovery with the addition of favipiravir to HCQ, but no significant difference in discharge, ICU admission, or hospitalization time.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
|
risk of ICU admission, 22.8% lower, RR 0.77, p = 1.00, treatment 3 of 34 (8.8%), control 4 of 35 (11.4%), NNT 38.
|
|
risk of no recovery, 87.5% lower, RR 0.12, p = 0.02, treatment 1 of 21 (4.8%), control 8 of 21 (38.1%), NNT 3.0, day 5, fever.
|
|
hospitalization time, 2.2% higher, relative time 1.02, p = 0.74, treatment 34, control 35.
|
|
risk of no hospital discharge, 2.9% higher, RR 1.03, p = 1.00, treatment 31 of 34 (91.2%), control 31 of 35 (88.6%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Delen et al., 31 Dec 2022, retrospective, Turkey, peer-reviewed, mean age 60.1, 8 authors, study period March 2020 - July 2020.
Contact: umutkasapoglu@gmail.com.
Effects of Hydroxychloroquine Plus Favipiravir Treatment on the Clinical Course and Biomarkers in Hospitalized COVID-19 Patients with Pneumonia
Acta clinica croatica, doi:10.20471/acc.2022.61.03.05
Background: The novel coronavirus disease 2019 (COVID-19) has a broad spectrum of clinical manifestations, the most common serious clinical manifestation of the coronavirus infection being pneumonia. Unfortunately, the optimal treatment approach is still uncertain. However, many studies have been conducted on the effectiveness of several medications in the treatment of COVID-19 infection. The aim of this study was to evaluate the effectiveness of the hydroxychloroquine (HCQ) + favipiravir (FAV) treatment regimen and HCQ alone by comparing the patient's clinical response and laboratory results on the fifth day of treatment in patients hospitalized due to COVID-19 infection. Patients and methods: This retrospective cohort study was conducted in Malatya Training and Research Hospital between March 2020 and July 2020. The study included 69 patients with confirmed COVID-19 with pneumonia. The patients were divided into 2 groups, those receiving HCQ alone and those receiving the HCQ + FAV combination. Results: A total of 69 patients were included in the study, and the mean age was 60.09±15.56 years. A statistically significant decrease was observed in C-reactive protein (CRP) levels, at the end of the fifth day, in patients who received HCQ + FAV treatment (p=0.002), whereas there was no decrease in CRP levels in patients who received HCQ treatment alone. In addition, an increase in lymphocyte count and a better fever response was observed at the end of the fifth day in patients who received HCQ + FAV (p=0.008). However, there was no statistical difference between both treatment regimens in terms of hospital stay and treatment results (p=0.008, p=0.744, p=0.517). Conclusion: Although the combination of HCQ + FAV treatment was observed to be effective on CRP levels and fever response in patients with COVID-19 pneumonia, there was no difference in terms of hospital stay and discharge.
Conflict of interest: The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Financial disclosure: None declared.
References
Ali, Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19, J Med Virol
Bateman, Sharpe, Jagger, Ellis, Sole-Violan et al., Erratum to: 36th International Symposium on Intensive Care and Emergency Medicine: Brussels, Crit Care
Cai, Yang, Liu, Chen, Shu et al., Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering (Beijing)
Cao, Liu, Xiong, Cai, Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: A systematic review and meta-analysis, J Med Virol
Chen, Hu, Zhang, Jiang, Han et al., Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial
Chen, Liu, Liu, Liu, Xu et al., A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19
Chen, Zhang, Huang, Yin, Cheng et al., Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial
Cortegiani, Ingoglia, Ippolito, Giarratano, Einav, A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19, J Crit Care
Dong, Cao, Lu, Zhang, Du et al., Eleven faces of coronavirus disease, Allergy
Dong, Hu, Gao, Discovering drugs to treat coronavirus disease 2019 (COVID-19), Drug Discov Ther
Du, Liang, Yang, Wang, Cao et al., Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study, Eur Respir J
Feng, Ling, Bai, Xie, Huang et al., COVID-19 with Different Severities: A Multicenter Study of Clinical Features, Am J Respir Crit Care Med
Gao, Ding, Dong, Zhang, Azkur et al., Risk factors for severe and critically ill COVID-19 patients: A review, Allergy
Gao, Tian, Yang, Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies, Biosci Trends
Grasselli, Zangrillo, Zanella, Antonelli, Cabrini et al., Baseline Characteristics and Outcomes of 1591 Patients Infected With Admitted to ICUs of the Lombardy Region, Italy, Jama
Guan, Ni, Hu, Liang, Ou et al., Clinical Characteristics of Coronavirus Disease 2019 in China, N Engl J Med
Harapan, Itoh, Yufika, Winardi, Keam et al., Coronavirus disease 2019 (COVID-19): A literature review, J Infect Public Health
He, Lu, Zhang, Fan, Xiong et al., The clinical course and its correlated immune status in COVID-19 pneumonia, J Clin Virol
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, Lancet
Ingraham, Lotfi-Emran, Thielen, Techar, Morris et al., None
Jung, Krieger, Hufert, Küpper, How we should respond to the Coronavirus SARS-CoV-2 outbreak: A German perspective, Clin Hemorheol Microcirc
Liang, Guan, Li, Li, Liang et al., Clinical characteristics and outcomes of hospitalised patients with COVID-19 treated in Hubei (epicentre) and outside Hubei (non-epicentre): a nationwide analysis of China, Eur Respir J
Mahevas, Tran, Roumier, Chabrol, Paule et al., Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data, BMJ
Mason, Pathogenesis of COVID-19 from a cell biology perspective, Eur Respir J
Mehta, Mazer-Amirshahi, Alkindi, Pourmand, Pharmacotherapy in COVID-19; A narrative review for emergency providers, Am J Emerg Med
Odabasi, Cinel, Consideration of Severe Coronavirus Disease 2019 As Viral Sepsis and Potential Use of Immune Checkpoint Inhibitors, Crit Care Explor
Ozger, Yildiz, Gaygisiz, Ugras, Demirbas et al., The factors predicting pneumonia in COVID-19 patients: preliminary results of a university hospital in Turkey, Turk J Med Sci
Ran, Chen, Wang, Wu, Zhang et al., Risk Factors of Healthcare Workers with Corona Virus Disease 2019: A Retrospective Cohort Study in a Designated Hospital of Wuhan in China, Clin Infect Dis
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area, Jama
Rothan, Byrareddy, The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak, J Autoimmun
Ruan, Yang, Wang, Jiang, Song, Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China, Intensive Care Med
Sadeghi-Haddad-Zavareh, Bayani, Shokri, Ebrahimpour, Babazadeh et al., C-Reactive Protein as a Prognostic Indicator in COVID-19 Patients. Interdiscip Perspect Infect Dis
Sanders, Monogue, Jodlowski, Cutrell, Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review, JAMA
Sažetak, Pluća, Delen, Gok, Kasapoglu et al., Tetik Pozadina: Bolest uzrokovana novim koronavirusom 2019 (COVID-19) ima širok spektar kliničkih manifestacija, pri čemu je upala pluća najčešća ozbiljna klinička manifestacija infekcije koronavirusom. Nažalost, optimalni pristup liječenju još uvijek nije jasno utvrđen. Ipak, provedeno je mnogo studija koje su istraživale učinkovitost raznih lijekova u liječenju infekcije COVID-19 virusom. Cilj ove studije bio je utvrditi učinkovitost liječenja kombinacijom hidroksiklorokina (HCQ) i favipiravira (FAV) te liječenja isključivo HCQ-om, uspoređivanjem kliničkog odgovora pacijenata na liječenje i njihovih laboratorijskih rezultata nakon pet dana liječenja u pacijenata hospitaliziranih zbog infekcije virusom COVID-19. Pacijenti i metode: Ova retrospektivna kohortna studija provedena je u Malatya Training and Research Hospital između ožujka 2020. i srpnja 2020. Uključivala je 69 pacijenata s potvrđenom infekcijom virusa COVID-19 sa upalom pluća. Pacijenti su podijeljeni u dvije skupine, od kojih je jedna primala samo HCQ, a druga kombinaciju HCQ + FAV. Rezultati: U studiju je uključeno ukupno 69 pacijenata s prosječnom dobi od 60,09±15,56 godina. Zamijećena je statistički značajno smanjenje u razini C-reaktivnog proteina (CRP) na kraju petog dana liječenja u pacijenata koji su primali HCQ + FAV (p=0,002), a nije bilo smanjenja u razini CRP-a u pacijenata koji su primali samo HCQ. Uz to, u pacijenata koji su primali HCQ + FAV zamijećeno je i povećanje u broju limfocita te bolji odgovor na vrućicu na kraju petog dana liječenja (p=0,008)
Shakoori, Hafeez, Malik, Could covid-19 be a hemoglobinopathy?, Acta Clinica Croatica
Sharifpour, Rangaraju, Liu, Alabyad, Nahab et al., C-Reactive protein as a prognostic indicator in hospitalized patients with COVID-19, PLoS One
Siddiqi, Mehra, COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal, J Heart Lung Transplant
Sun, Wang, Cai, Hu, Chen et al., Cytokine storm intervention in the early stages of COVID-19 pneumonia, Cytokine Growth Factor Rev
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Wang, Hu, Hu, Zhu, Liu et al., Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, JAMA
Yang, Zheng, Gou, Pu, Chen et al., Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis, Int J Infect Dis
Yavuz, Unal, Antiviral treatment of COVID-19, Turk J Med Sci
Zhang, Hou, Ma, Li, Xue et al., The common risk factors for progression and mortality in COVID-19 patients: a meta-analysis, Arch Virol
Zheng, Peng, Xu, Zhao, Liu et al., Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis, J Infect
Zhou, Dai, Tong, COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression, J Antimicrob Chemother
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet
DOI record:
{
"DOI": "10.20471/acc.2022.61.03.05",
"ISSN": [
"0353-9466"
],
"URL": "http://dx.doi.org/10.20471/acc.2022.61.03.05",
"author": [
{
"affiliation": [],
"family": "Delen",
"given": "Leman Acun",
"sequence": "first"
}
],
"container-title": "Acta clinica croatica",
"container-title-short": "ACC",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
5,
24
]
],
"date-time": "2023-05-24T07:14:04Z",
"timestamp": 1684912444000
},
"deposited": {
"date-parts": [
[
2023,
5,
24
]
],
"date-time": "2023-05-24T07:16:39Z",
"timestamp": 1684912599000
},
"indexed": {
"date-parts": [
[
2023,
5,
25
]
],
"date-time": "2023-05-25T04:30:43Z",
"timestamp": 1684989043684
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022
]
]
},
"link": [
{
"URL": "https://hrcak.srce.hr/file/428402",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "8565",
"original-title": [],
"prefix": "10.20471",
"published": {
"date-parts": [
[
2022
]
]
},
"published-print": {
"date-parts": [
[
2022
]
]
},
"publisher": "Sestre Milosrdnice University Hospital Center (KBC Sestre milosrdnice)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://hrcak.srce.hr/clanak/428402"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Effects of Hydroxychloroquine Plus Favipiravir Treatment on the Clinical Course and Biomarkers in Hospitalized COVID-19 Patients with Pneumonia",
"type": "journal-article"
}