Sotrovimab for Pre-exposure Prophylaxis against SARS-CoV2 in a Vulnerable Patient Population: Results from the PROTECT-V trial
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.340, PROTECT-V, Jan 2026
Sotrovimab for COVID-19
45th treatment shown to reduce risk in
August 2022, now with p = 0.00048 from 29 studies, recognized in 42 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
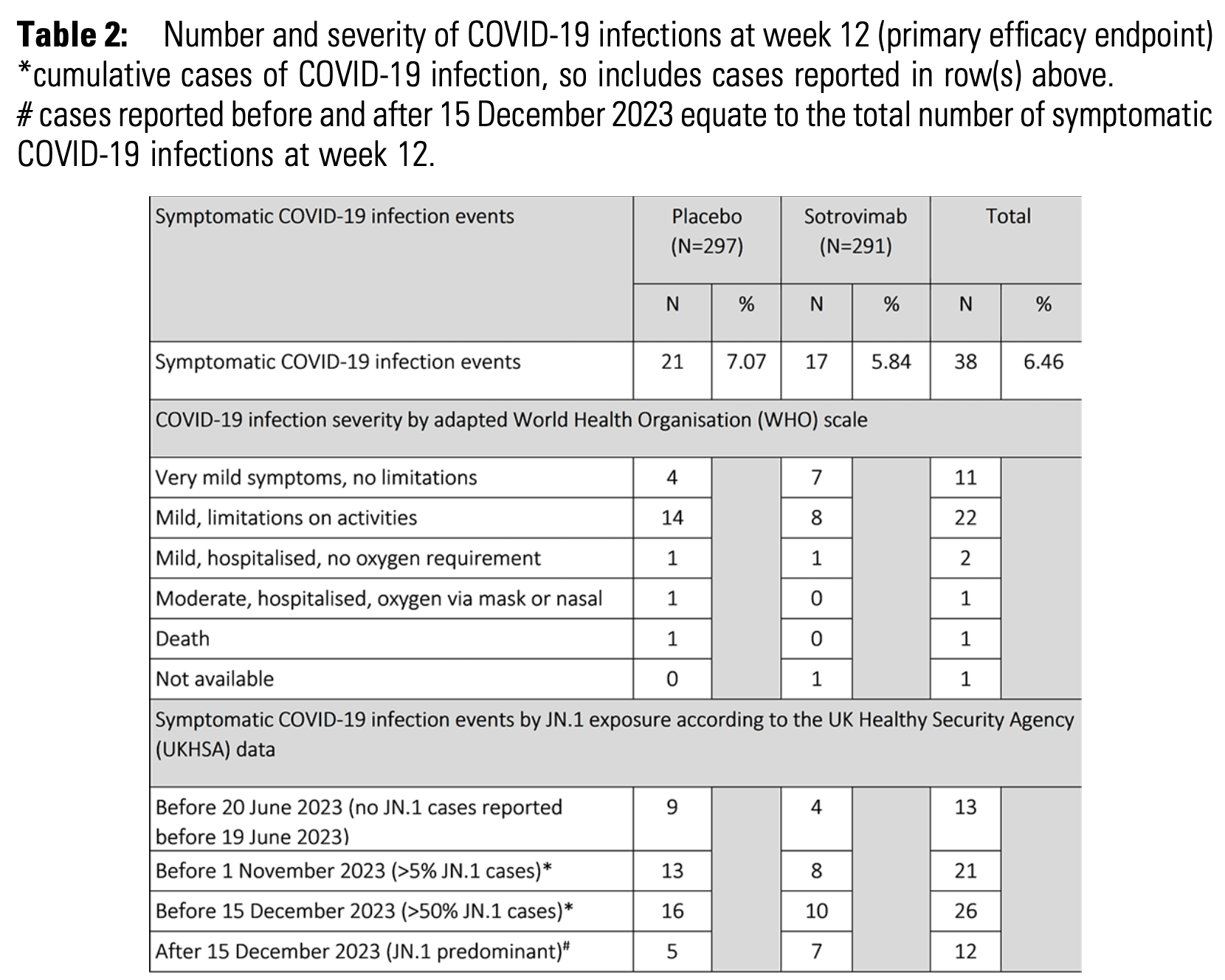
RCT 619 vulnerable patients showing no significant difference in symptomatic COVID-19 infections at 12 weeks with sotrovimab pre-exposure prophylaxis.
Efficacy is variant dependent. In Vitro studies predict lower efficacy for BA.11-3, BA.4, BA.54, XBB.1.9.3, XBB.1.5.24, XBB.2.9, CH.1.15, and no efficacy for BA.26, XBB, XBB.1.5, ХВВ.1.9.17, XBB.1.16, BQ.1.1.45, and CL.15. US EUA has been revoked.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments8.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 66.4% lower, RR 0.34, p = 1.00, treatment 0 of 291 (0.0%), control 1 of 297 (0.3%), NNT 297, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of hospitalization, 66.0% lower, RR 0.34, p = 0.62, treatment 1 of 291 (0.3%), control 3 of 297 (1.0%), NNT 150.
|
|
risk of symptomatic case, 20.0% lower, RR 0.80, p = 0.51, treatment 17 of 291 (5.8%), control 21 of 297 (7.1%), NNT 81, adjusted per study.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
6.
Zhou et al., SARS-CoV-2 Omicron BA.2 Variant Evades Neutralization by Therapeutic Monoclonal Antibodies, bioRxiv, doi:10.1101/2022.02.15.480166.
Chen-Xu et al., 11 Jan 2026, Double Blind Randomized Controlled Trial, placebo-controlled, United Kingdom, peer-reviewed, median age 64.4, 12 authors, study period August 2022 - May 2024, PROTECT-V trial.
P-112. Sotrovimab for Pre-exposure Prophylaxis against SARS-CoV2 in a Vulnerable Patient Population: Results from the PROTECT-V trial
max and AUC increased dose-proportionally. The median T max was 173-338 hours; and the mean t½ was 602-930 hours (Table 2 ). The median time to achieve anti-tetanus antibody titers ≥0.01 IU/mL (protective threshold) was < 10 hours in both the100 and 250 μg/kg dose cohorts. At 105 days post-dosing, more than 66% participants in the 100 μg/kg cohort, and 100% in the 250 μg/kg cohort maintained antibody titers ≥0.01 IU/mL. The serum antibody titer-time profiles are shown in Figure 1&2 .
DOI record:
{
"DOI": "10.1093/ofid/ofaf695.340",
"ISSN": [
"2328-8957"
],
"URL": "http://dx.doi.org/10.1093/ofid/ofaf695.340",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Despite introduction of vaccination against SARS CoV-2, there remains a need for pre-exposure prophylaxis in patients that mount suboptimal vaccine responses. Sotrovimab is a recombinant human monoclonal antibody directed against the spike protein of SARS-CoV-2. The COMET-ICE study demonstrated that 500mg intravenous (IV) sotrovimab significantly reduced all-cause hospitalization or death in high-risk non-hospitalised patients with mild/moderate COVID-19 infection.Table 2:Number and severity of COVID-19 infections at week 12 (primary efficacy endpoint)*cumulative cases of COVID-19 infection, so includes cases reported in row(s) above.# cases reported before and after 15 December 2023 equate to the total number of symptomatic COVID-19 infections at week 12.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>PROTECT-V is a platform trial evaluting prophylactic interventions against SARS-CoV2 infection in vulnerable adult patients at high risk of COVID-19 infection and its complications, including dialysis patients, transplant recipients, those with autoimmune diseases, underlying immunodeficiency or haematological/oncological diagnoses.</jats:p>\n <jats:p>Sotrovimab was the second agent added to the platform. Participants were randomized 1:1 to one dose of IV sotrovimab 2000mg or matched placebo. The primary endpoint was confirmed symptomatic COVID-19 infection at week 12.Table 3:Safety and Tolerability of 2000mg intravenous Sotrovimab</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>619 (314 placebo, 305 sotrovimab) patients in the UK were randomized between August 2022 and May 2024. 588 (297 placebo, 291 sotrovimab) received investigational medicinal product infusion. Overall, median age was 64.4 years and 50.5% were female (see Table 1: Baseline characteristics).</jats:p>\n <jats:p>At week 12, 21 symptomatic COVID-19 infections were observed in the placebo group and 17 in the sotrovimab group with a risk ratio of 0.80 (95% CI 0.42– 1.53) adjusting for age and disease group. 4/38 (10.5%) patients were hospitalised (3 placebo, 1 sotrovimab). In view of the 250-fold reduction in EC50 with sotrovimab on pseudovirus testing for JN.1, a pre-specified efficacy analysis according to exposure period was performed (Table 2).</jats:p>\n <jats:p>Headache, dizziness and skin symptoms were the most common adverse events reported within 24 hours of infusion (Table 3).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>Although safe and well tolerated, 2000mg sotrovimab did not demonstrate benefit over placebo for the prevention of symptomatic SARS-CoV2 infection at 12 weeks. There was potential signal of benefit for sotrovimab before the emergence of the JN.1 variant in late 2023 that rapidly became the dominant circulating variant in the UK at that time.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Disclosures</jats:title>\n <jats:p>Michael Chen-Xu, MBChB, MRCP, MPH, GlaxoSmithKline: Grant/Research Support Davinder Dosanjh, n/a, Astrazeneca: Employee Jennifer Han, MD, GlaxoSmithKline: Employee Thomas F. Hiemstra, n/a, GlaxoSmithKline: Stocks/Bonds (Public Company)|Novartis: Employee|Novartis: Stocks/Bonds (Public Company) Alex G. Richter, n/a, CSL Behring: Honoraria Rona M. Smith, MD MRCP, AstraZeneca: Advisor/Consultant|AstraZeneca: Honoraria|GlaxoSmithKline: Grant/Research Support|Vifor Pharma: Honoraria</jats:p>\n </jats:sec>",
"article-number": "ofaf695.340",
"author": [
{
"affiliation": [
{
"name": "University of Cambridge , Cambridge, England ,",
"place": [
"United Kingdom"
]
}
],
"family": "Chen-Xu",
"given": "Michael",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Cambridge University Hospitals NHS Foundation Trust , Cambridge, England ,",
"place": [
"United Kingdom"
]
}
],
"family": "Qian",
"given": "Wendi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Cambridge , Cambridge, England ,",
"place": [
"United Kingdom"
]
}
],
"family": "Kamelian",
"given": "Kimia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Cambridge , Cambridge, England ,",
"place": [
"United Kingdom"
]
}
],
"family": "Trivioli",
"given": "Giorgio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Birmingham , Birmingham, Northern Ireland ,",
"place": [
"United Kingdom"
]
}
],
"family": "Dosanjh",
"given": "Davinder",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Cambridge University Hospitals NHS Foundation Trust , Cambridge, England ,",
"place": [
"United Kingdom"
]
}
],
"family": "Dowling",
"given": "Francis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Cambridge University Hospitals NHS Foundation Trust , Cambridge, England ,",
"place": [
"United Kingdom"
]
}
],
"family": "Adhikari",
"given": "Rakshya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "GlaxoSmithKline , Collegeville, Pennsylvania"
}
],
"family": "Han",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Cambridge University Hospitals NHS Foundation Trust , Cambridge, England ,",
"place": [
"United Kingdom"
]
}
],
"family": "Hiemstra",
"given": "Thomas F",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Birmingham , Birmingham, Northern Ireland ,",
"place": [
"United Kingdom"
]
}
],
"family": "Richter",
"given": "Alex G",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Cambridge , Cambridge, England ,",
"place": [
"United Kingdom"
]
}
],
"family": "Gupta",
"given": "Ravindra K",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Cambridge , Cambridge, England ,",
"place": [
"United Kingdom"
]
}
],
"family": "Smith",
"given": "Rona M",
"sequence": "additional"
}
],
"container-title": "Open Forum Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2026,
1,
12
]
],
"date-time": "2026-01-12T06:45:07Z",
"timestamp": 1768200307000
},
"deposited": {
"date-parts": [
[
2026,
1,
12
]
],
"date-time": "2026-01-12T06:45:07Z",
"timestamp": 1768200307000
},
"indexed": {
"date-parts": [
[
2026,
1,
12
]
],
"date-time": "2026-01-12T09:50:44Z",
"timestamp": 1768211444367,
"version": "3.49.0"
},
"is-referenced-by-count": 0,
"issue": "Supplement_1",
"issued": {
"date-parts": [
[
2026,
1
]
]
},
"journal-issue": {
"issue": "Supplement_1",
"published-print": {
"date-parts": [
[
2026,
1,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 11,
"start": {
"date-parts": [
[
2026,
1,
12
]
],
"date-time": "2026-01-12T00:00:00Z",
"timestamp": 1768176000000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/ofid/article-pdf/13/Supplement_1/ofaf695.340/66345257/ofaf695.340.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/article-pdf/13/Supplement_1/ofaf695.340/66345257/ofaf695.340.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2026,
1
]
]
},
"published-online": {
"date-parts": [
[
2026,
1,
11
]
]
},
"published-other": {
"date-parts": [
[
2026,
1
]
]
},
"published-print": {
"date-parts": [
[
2026,
1,
11
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ofid/article/doi/10.1093/ofid/ofaf695.340/8420411"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "P-112. Sotrovimab for Pre-exposure Prophylaxis against SARS-CoV2 in a Vulnerable Patient Population: Results from the PROTECT-V trial",
"type": "journal-article",
"volume": "13"
}
