Safety and Antiviral Efficacy of a Broad-Spectrum siRNA SNS812 Targeting SARS-CoV-2: A Phase II Trial
et al., CROI 2025, Mar 2025
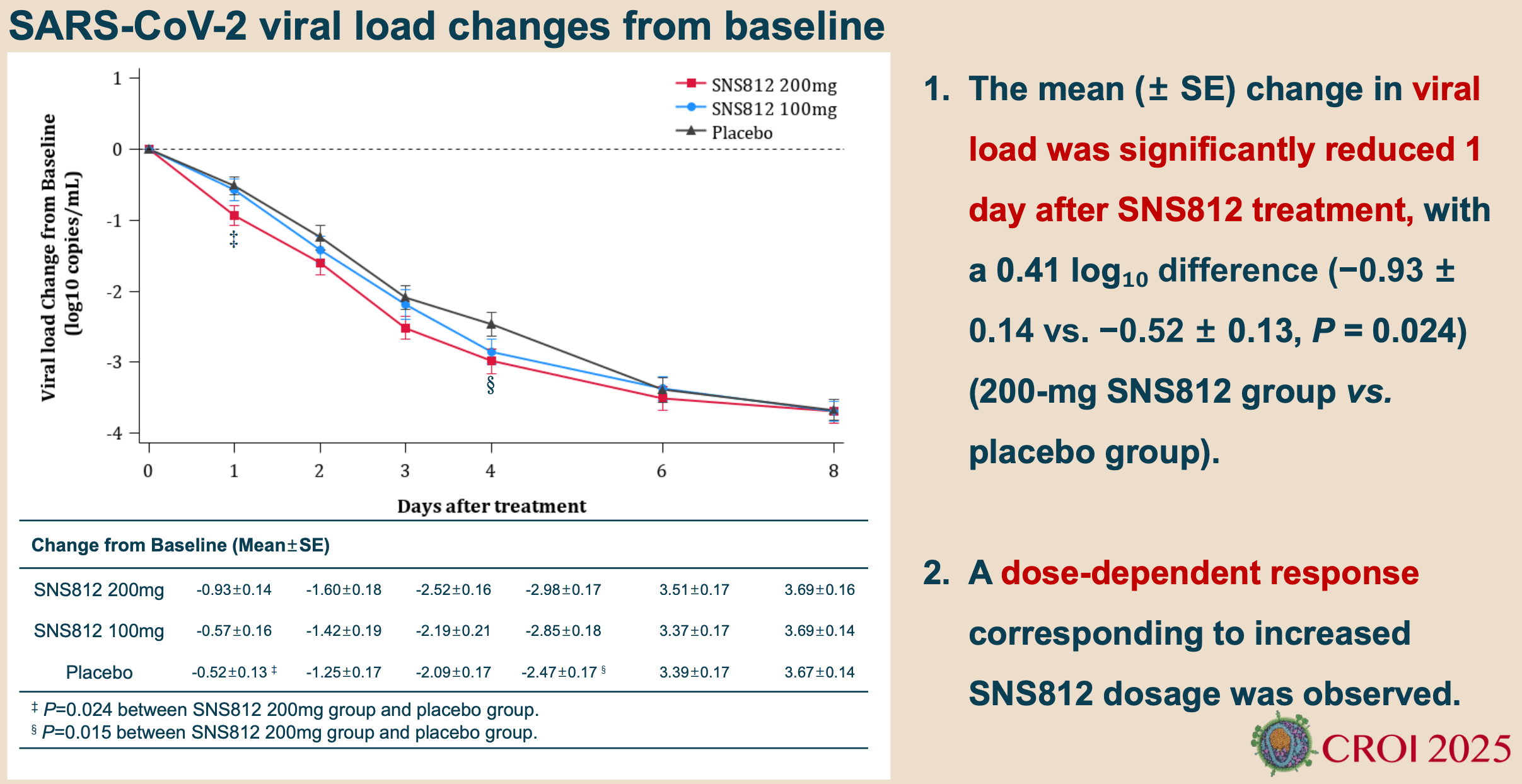
RCT 135 patients with mild to moderate COVID-19 showing significant efficacy of aerosolized siRNA SNS812 in reducing viral load and accelerating symptom resolution. The trial randomized patients to 200mg, 100mg, or placebo arms, with treatment initiated within 3 days of symptom onset. The 200mg group demonstrated significantly faster viral clearance and greater viral load reduction starting from day 1. Symptom improvement showed dose-dependent effects with significant reduction in time to resolution for 6 of 14 symptoms, including shortness of breath and loss of taste/smell. Authors do not provide enough information on symptomatic outcomes for meta analysis. The siRNA targets a highly conserved region of the viral RNA-dependent RNA polymerase gene and showed efficacy against multiple Omicron variants.
|
viral load, 17.1% lower, relative load 0.83, p = 0.01, treatment mean 2.98 (±1.14) n=45, control mean 2.47 (±1.14) n=45, day 4.
|
|
viral load, 13.3% lower, relative load 0.87, p = 0.13, treatment mean 2.85 (±1.19) n=44, control mean 2.47 (±1.14) n=45, day 4.
|
|
time to viral-, 19.4% lower, relative time 0.81, p = 0.007, treatment 45, control 45.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Chang et al., 11 Mar 2025, Double Blind Randomized Controlled Trial, Taiwan, peer-reviewed, 4 authors, study period 30 October, 2023 - 12 August, 2024, 100 mg$c$ mid-recovery.
Contact: erdrcsy@gmail.com.
CDC, USA) LP.8.1 XEC KP.3 KW.1.1 JN.1 KP.2 EG.5 XBB.1.9 XBB.1.16 XBB XBC ~ 2/22/2025 (NSW, Australia) * Includes old age ≧ 65 years old, overweight or obesity, diabetes mellitus, hypertension, coronary artery disease, chronic liver disease, chronic kidney disease, asthma, thalassemia and depression. † Overweight was defined as a BMI of 25 to 29, and obesity was defined as a BMI of 30 or greater at the day of evaluation for eligibility. ‡ Includes feeling hot or feverish, chills or shivers, low energy or tiredness, headache, muscle or body aches, stuffy or runny nose, sore or dry throat, cough, shortness of breath, nausea, vomiting, diarrhea, loss of smell, and loss of taste, * P=0.036 between SNS812 200mg group and SNS812 100mg group. † P=0.006 between SNS812 200mg group and placebo group.
Thank You for Listening! erdrcsy@gmail.com
Tseng, Wu Chien-Hao Lin, Sheng Chyi-Feng, Chen, Shao et al., None