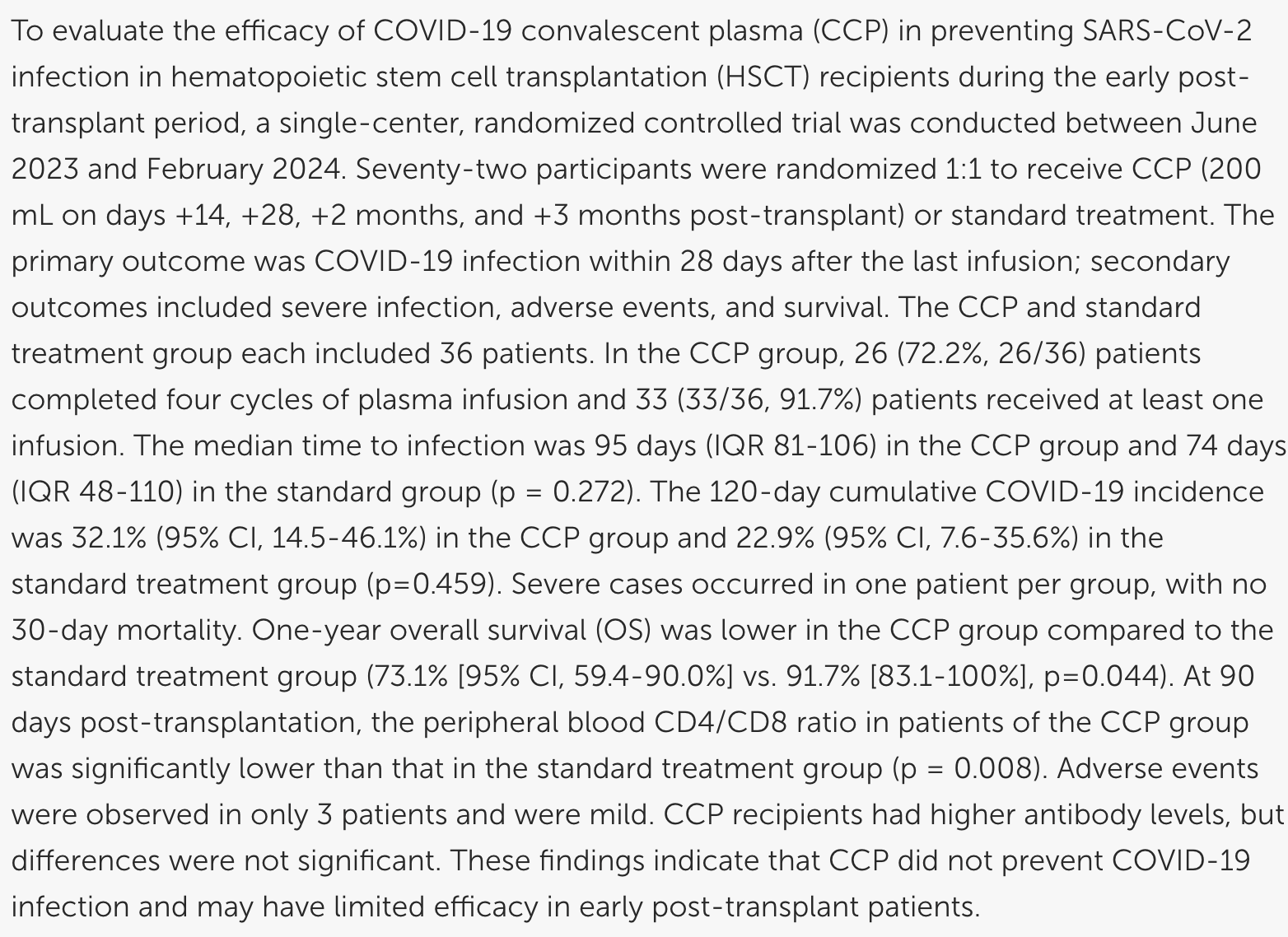
Convalescent Plasma Therapy for COVID-19 Prophylaxis in Adults Early Post-Hematopoietic Stem Cell Transplantation: One-Year Outcomes from a Randomized Controlled Trial
et al., Frontiers in Immunology, doi:10.3389/fimmu.2025.162677, Nov 2025
RCT 72 hematopoietic stem cell transplantation (HSCT) recipients, showing higher one-year mortality with COVID-19 convalescent plasma prophylaxis treatment.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
|
risk of death, 224.1% higher, RR 3.24, p = 0.04, treatment 36, control 36, day 365.
|
|
risk of severe case, no change, RR 1.00, p = 1.00, treatment 1 of 36 (2.8%), control 1 of 36 (2.8%), day 120.
|
|
risk of case, 40.2% higher, RR 1.40, p = 0.46, treatment 36, control 36, day 120.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Cao et al., 11 Nov 2025, Randomized Controlled Trial, China, peer-reviewed, 22 authors, study period June 2023 - February 2024.
Contact: sunjiali@ihcams.ac.cn, doctor_eljiang@163.com.