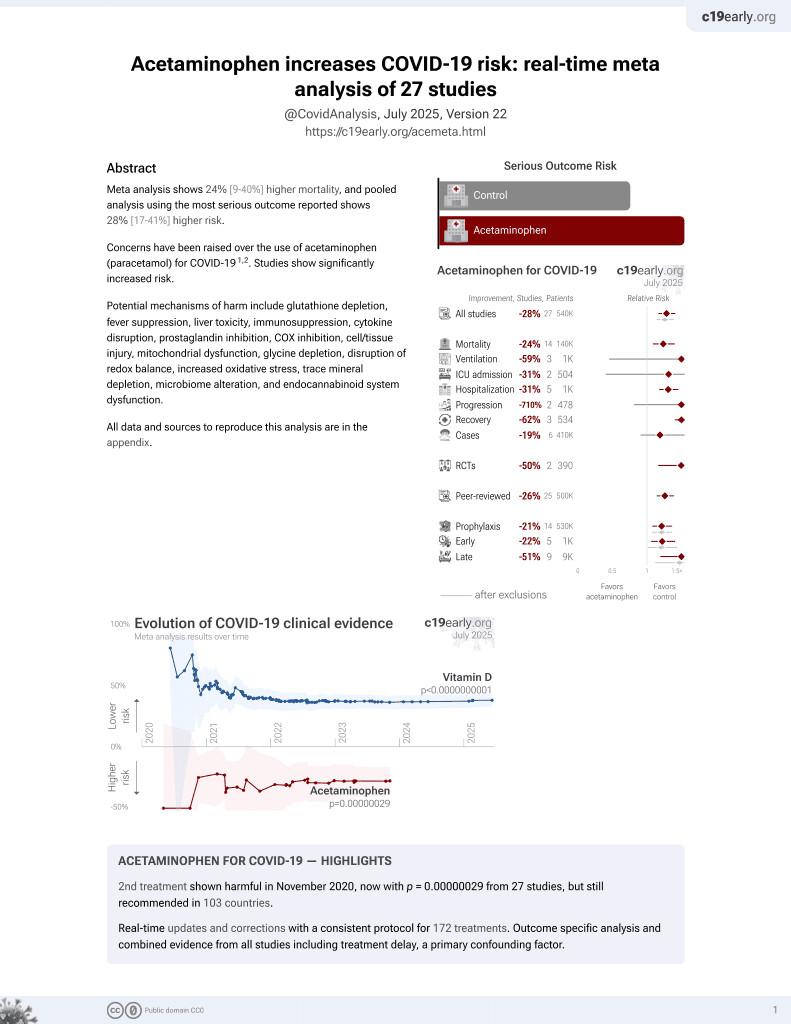
Chronic use of non-steroidal anti-inflammatory drugs (NSAIDs) or acetaminophen and relationship with mortality among United States Veterans after testing positive for COVID-19
et al., PLOS ONE, doi:10.1371/journal.pone.0267462, May 2022
2nd treatment shown to increase risk in
November 2020, now with p = 0.00000029 from 27 studies, but still recommended in 103 countries.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 28,856 COVID-19 patients in the USA, showing no significant difference in mortality for chronic acetaminophen use vs. sporadic NSAID use. Since acetaminophen is available OTC and authors only tracked prescriptions, many patients classified as sporadic users may have been chronic users.
Acetaminophen is also known as paracetamol, Tylenol, Panadol, Calpol, Tempra, Calprofen, Doliprane, Efferalgan, Grippostad C, Dolo, Acamol, Fevadol, Crocin, and Perfalgan.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
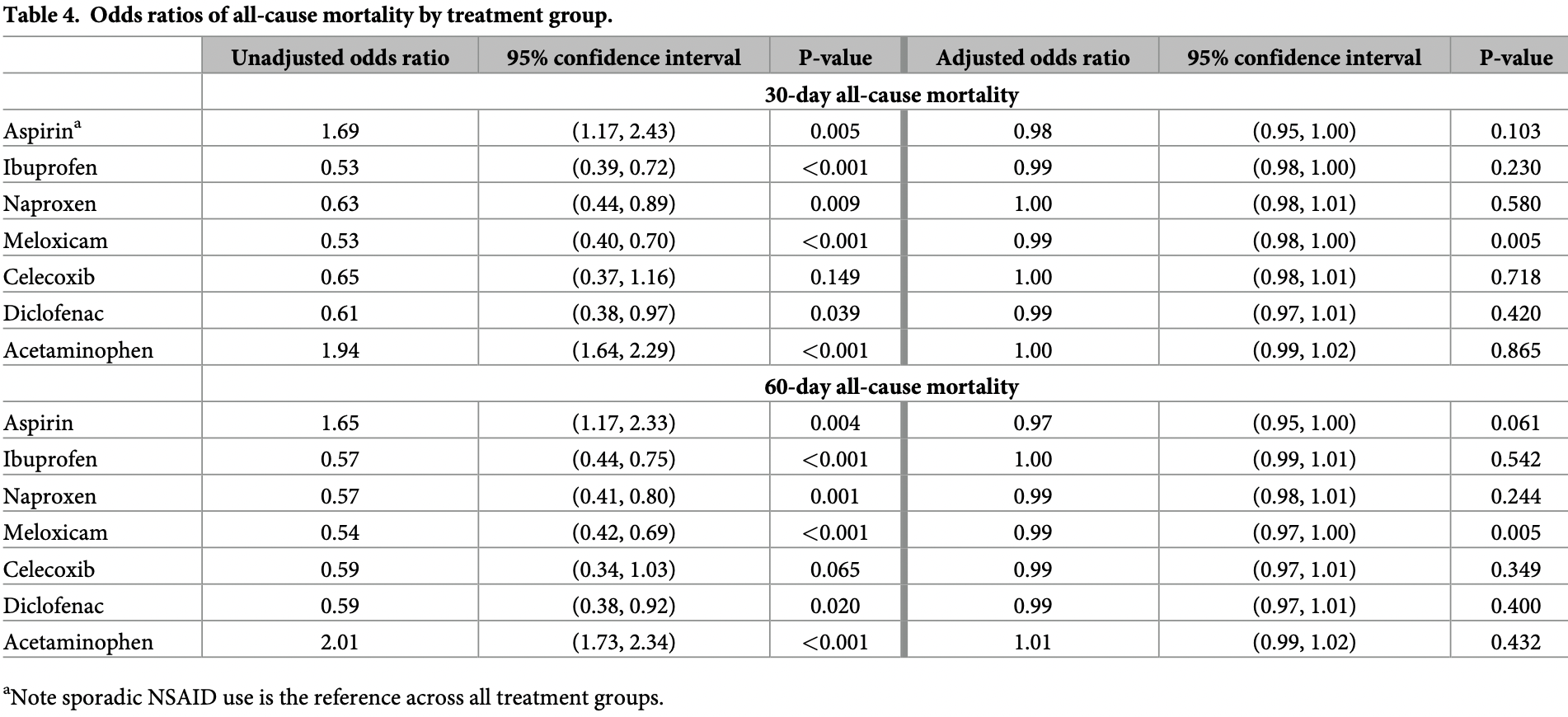
risk of death, 1.0% higher, OR 1.01, p = 0.43, treatment 2,074, control 20,311, adjusted per study, propensity score weighting, multivariable, day 60, RR approximated with OR.
|
|
risk of death, no change, OR 1.00, p = 0.86, treatment 2,074, control 20,311, adjusted per study, propensity score weighting, multivariable, day 30, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Campbell et al., 5 May 2022, retrospective, USA, peer-reviewed, 4 authors, study period 2 March, 2020 - 14 December, 2020.
Contact: heather.campbell@va.gov, virec@va.gov.
Chronic use of non-steroidal anti-inflammatory drugs (NSAIDs) or acetaminophen and relationship with mortality among United States Veterans after testing positive for COVID-19
PLOS ONE, doi:10.1371/journal.pone.0267462
Non-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen are among the mostfrequently used medications. Although these medications have different mechanisms of action, they have similar indications and treatment duration has been positively correlated with cardiovascular risk although the degree of risk varies by medication. Our objective was to study treatment effects of chronic use of individual NSAID medications and acetaminophen on all-cause mortality among patients who tested positive for COVID-19 while accounting for adherence. We used the VA national datasets in this retrospective cohort study to differentiate between sporadic and chronic medication use: sporadic users filled an NSAID within the last year, but not recently or regularly. Using established and possible risk factors for severe COVID-19, we used propensity scores analysis to adjust for differences in baseline characteristics between treatment groups. Then, we used multivariate logistic regression incorporating inverse propensity score weighting to assess mortality. The cohort consisted of 28,856 patients. Chronic use of aspirin, ibuprofen, naproxen, meloxicam, celecoxib, diclofenac or acetaminophen was not associated with significant differences in mortality at 30 days (
take these medications chronically is no different than patients who sporadically used NSAIDs. This information can also be used by clinicians caring for patients as they make clinical treatment decisions based on their risk assessment. Our attempt to address this was by controlling the month or season in which patients tested positive: with an unadjusted OR = 0.90 and OR = 0.91 for 30-day and 60-day mortality, respectively, month did not meet the criteria for a moderate association to be included in the final propensity score-adjusted model. With season having OR = 0.66 and OR = 0.68 for 30-and 60-day mortality, respectively, it also did not meet the criteria of OR = 0.50 to be included.
References
Andersohn, Suissa, Garbe, Use of first-and second-generation cyclooxygenase-2-selective nonsteroidal antiinflammatory drugs and risk of acute myocardial infarction, Circulation, doi:10.1161/CIRCULATIONAHA.105.602425
Arnold, CDW date of death analysis and vital status file comparison update
Asadi, Sayar, Radmanesh, Naghshi, Mousaviasl et al., Efficacy of naproxen in the management of patients hospitalized with COVID-19 infection: a randomized, double-blind, placebocontrolled clinical trial, Diabetes Metab Syndr, doi:10.1016/j.dsx.2021.102319
Austin, Grootendorst, Anderson, A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study, Stat Med, doi:10.1002/sim.2580
Bhala, Emberson, Merhi, Abramson, Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials, Lancet, doi:10.1016/S0140-6736%2813%2960900-9
Bruce, Barlow-Pay, Short, Vilches-Moraga, Price et al., Prior routine use of non-steroidal anti-inflammatory drugs (NSAIDs) and important outcomes in hospitalised patients with COVID-19, J Clin Med
Chan, Manson, Albert, Chae, Rexrode et al., Nonsteroidal antiinflammatory drugs, acetaminophen, and the risk of cardiovascular events, Circulation, doi:10.1161/CIRCULATIONAHA.105.595793
Chandan, Zemedikun, Thayakaran, Byne, Dhalla et al., Arthritis Rheumatol, doi:10.1002/art.41593
Chandan, Zemedikun, Thayakaran, Byne, Dhalla et al., Non-steroidal anti-inflammatory drugs and susceptibility to COVID-19, Arthritis Rheumatol, doi:10.1002/art.41593
Chmiel, Konstan, Accurso, Lymp, Mayer-Hamblett et al., Use of ibuprofen to assess inflammatory biomarkers in induced sputum: Implications for clinical trials in cystic fibrosis, J Cyst Fibros, doi:10.1016/j.jcf.2015.03.007
Chow, Khanna, Kethireddy, Yamane, Levine et al., Aspirin use is associated with decreased mechanical ventilation, intensive care unit admission, and in-hospital mortality in hospitalized patients with Coronavirus Disease 2019, Anesth Analg, doi:10.1213/ANE.0000000000005292
Eeren, Spreeuwenberg, Bartak, De Rooij, Busschbach, Estimating subgroup effects using the propensity score method: a practical application in outcomes research, Med Care, doi:10.1097/MLR.0000000000000325
Esba, Alqahtani, Thomas, Shamas, Alswaidan et al., Ibuprofen and NSAID use in COVID-19 infected patients is not associated with worse outcomes: a prospective cohort study, Infect Dis Ther, doi:10.1007/s40121-020-00363-w
Fang, Karakiulakis, Roth, Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?, Lancet Respir Med, doi:10.1016/S2213-2600%2820%2930116-8
Fuster, Sweeney, Aspirin: a historical and contemporary therapeutic overview, Circulation, doi:10.1161/CIRCULATIONAHA.110.963843
Gallelli, Galasso, Falcone, Southworth, Greco et al., The effects of nonsteroidal anti-inflammatory drugs on clinical outcomes, synovial fluid cytokine concentration and signal transduction pathways in knee osteoarthritis. A randomized open label trial, Osteoarthritis Cartilage, doi:10.1016/j.joca.2013.06.026
Giollo, Adami, Gatti, Idolazzi, Rossini, Coronavirus disease 19 (Covid-19) and non-steroidal anti-inflammatory drugs (NSAID), Ann Rheum Dis, doi:10.1136/annrheumdis-2020-217598
Helin-Salmivaara, Virtanen, Vesalainen, Gronroos, Klaukka et al., NSAID use and the risk of hospitalization for first myocardial infarction in the general population: a nationwide case-control study from Finland, Eur Heart J, doi:10.1093/eurheartj/ehl053
Hong, Chen, You, Tan, Wu et al., Celebrex adjuvant therapy on Coronavirus Disease 2019: an experimental study, Front Pharmacol, doi:10.3389/fphar.2020.561674
Jeong, Lee, Shin, Choe, Filion et al., Association between NSAIDs use and adverse clinical outcomes among adults hospitalized with COVID-19 in South Korea: A nationwide study, Clin Infect Dis, doi:10.1093/cid/ciaa1056
Kragholm, Gerds, Fosbol, Andersen, Phelps et al., Association between prescribed ibuprofen and severe COVID-19 infection: a nationwide register-based cohort study, Clin Transl Sci, doi:10.1111/cts.12904
Lund, Kristensen, Reilev, Christensen, Thomsen et al., Adverse outcomes and mortality in users of non-steroidal anti-inflammatory drugs who tested positive for SARS-CoV-2: A Danish nationwide cohort study, PLoS Med, doi:10.1371/journal.pmed.1003308
Meizlish, Goshua, Liu, Fine, Amin et al., Intermediate-dose anticoagulation, aspirin, and in-hospital mortality in COVID-19: A propensity score-matched analysis, Am J Hematol, doi:10.1002/ajh.26102
Nguyen, Collins, Spence, Daures, Devereaux et al., Double-adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance, BMC Med Res Methodol, doi:10.1186/s12874-017-0338-0
Osborne, Veigulis, Arreola, Mahajan, Roosli et al., Association of mortality and aspirin prescription for COVID-19 patients at the Veterans Health Administration, PLoS One, doi:10.1371/journal.pone.0246825
Petersen, Porter, Gruber, Wang, Van Der Laan, Diagnosing and responding to violations in the positivity assumption, Stat Methods Med Res, doi:10.1177/0962280210386207
Rosenbaum, Propsensity score
Sahai, Bhandari, Godwin, Mcintyre, Chung et al., Effects of aspirin on shortterm outcomes in hospitalized patients with COVID-19, Vasc Med, doi:10.1177/1358863X211012754
Sahai, Bhandari, Koupenova, Freedman, Godwin et al., SARS-CoV-2 receptors are expressed on human platelets and the effect of aspirin on clinical outcomes in COVID-19 patients, Res Sq, doi:10.21203/rs.3.rs-119031/v1
Schmidt, Christiansen, Mehnert, Rothman, Sorensen, Non-steroidal anti-inflammatory drug use and risk of atrial fibrillation or flutter: population based case-control study, BMJ, doi:10.1136/bmj.d3450
Schmidt, Lamberts, Olsen, Fosboll, Niessner et al., Cardiovascular safety of non-aspirin non-steroidal anti-inflammatory drugs: review and position paper by the working group for Cardiovascular Pharmacotherapy of the European Society of Cardiology, Eur Heart J, doi:10.1093/eurheartj/ehv505
Son, Noh, Lee, Seo, Park et al., Effect of aspirin on coronavirus disease 2019: a nationwide case-control study in South Korea, Medicine, doi:10.1097/MD.0000000000026670
United, States Food and Drug Administration. FDA advises patients on use of non-steroidal anti-inflammatory drugs (NSAIDs) for COVID-19
Wong, Mackenna, Morton, Schultze, Walker et al., Use of non-steroidal anti-inflammatory and risk of death from COVID-19: an OpenSAFELY cohort analysis based on two cohorts, BMJ, doi:10.1136/annrheumdis-2020-219517
Wong, Wang, Liu, Hebert, Maciejewski, Do Veterans Health Administration enrollees generalize to other populations?, Med Care Res Rev, doi:10.1177/1077558715617382
Zhao, Gao, Dai, Treggiari, Deshpande et al., Treatments associated with lower mortality among critially ill COVID-19 patients: a retrospective cohort study, Anesthesiology, doi:10.1097/ALN.0000000000003999
DOI record:
{
"DOI": "10.1371/journal.pone.0267462",
"ISSN": [
"1932-6203"
],
"URL": "http://dx.doi.org/10.1371/journal.pone.0267462",
"abstract": "<jats:p>Non-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen are among the most-frequently used medications. Although these medications have different mechanisms of action, they have similar indications and treatment duration has been positively correlated with cardiovascular risk although the degree of risk varies by medication. Our objective was to study treatment effects of chronic use of individual NSAID medications and acetaminophen on all-cause mortality among patients who tested positive for COVID-19 while accounting for adherence. We used the VA national datasets in this retrospective cohort study to differentiate between sporadic and chronic medication use: sporadic users filled an NSAID within the last year, but not recently or regularly. Using established and possible risk factors for severe COVID-19, we used propensity scores analysis to adjust for differences in baseline characteristics between treatment groups. Then, we used multivariate logistic regression incorporating inverse propensity score weighting to assess mortality. The cohort consisted of 28,856 patients. Chronic use of aspirin, ibuprofen, naproxen, meloxicam, celecoxib, diclofenac or acetaminophen was not associated with significant differences in mortality at 30 days (OR = 0.98, 95% CI: 0.95–1.00; OR = 0.99, 95% CI: 0.98–1.00; OR = 1.00, 95% CI: 0.98–1.01; OR = 0.99, 95% CI: 0.98–1.00; OR = 1.00, 95% CI: 0.98–1.01; OR = 0.99, 95% CI: 0.97–1.01; and OR = 1.00, 95% CI: 0.99–1.02, respectively) nor at 60 days (OR = 0.97, 95% CI: 0.95–1.00; OR = 1.00, 95% CI: 0.99–1.01; OR = 0.99, 95% CI: 0.98–1.01; OR = 0.99, 95% CI: 0.97–1.00; OR = 0.99, 95% CI: 0.97–1.01; OR = 0.99, 95% CI: 0.97–1.01; and OR = 1.01, 95% CI: 0.99–1.02, respectively). Although the study design cannot determine causality, the study should assure patients as it finds no association between mortality and chronic use of these medications compared with sporadic NSAID use among those infected with COVID-19.</jats:p>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-6401-9240",
"affiliation": [],
"authenticated-orcid": true,
"family": "Campbell",
"given": "Heather M.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Murata",
"given": "Allison E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Conner",
"given": "Todd A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fotieo",
"given": "Greg",
"sequence": "additional"
}
],
"container-title": "PLOS ONE",
"container-title-short": "PLoS ONE",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"www.plosone.org"
]
},
"created": {
"date-parts": [
[
2022,
5,
5
]
],
"date-time": "2022-05-05T18:01:42Z",
"timestamp": 1651773702000
},
"deposited": {
"date-parts": [
[
2022,
5,
5
]
],
"date-time": "2022-05-05T18:03:06Z",
"timestamp": 1651773786000
},
"editor": [
{
"affiliation": [],
"family": "Lazzeri",
"given": "Chiara",
"sequence": "first"
}
],
"funder": [
{
"DOI": "10.13039/100012462",
"doi-asserted-by": "publisher",
"name": "Office of Academic Affiliations, Department of Veterans Affairs"
},
{
"name": "Veterans Health Administration"
},
{
"name": "New Mexico VA Health Care System"
},
{
"name": "Raymond G. Murphy VA Medical Center"
},
{
"name": "Cooperative Studies Program Clinical Research Pharmacy Coordinating Center"
}
],
"indexed": {
"date-parts": [
[
2022,
5,
6
]
],
"date-time": "2022-05-06T10:40:56Z",
"timestamp": 1651833656310
},
"is-referenced-by-count": 0,
"issue": "5",
"issued": {
"date-parts": [
[
2022,
5,
5
]
]
},
"journal-issue": {
"issue": "5",
"published-online": {
"date-parts": [
[
2022,
5,
5
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/publicdomain/zero/1.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
5,
5
]
],
"date-time": "2022-05-05T00:00:00Z",
"timestamp": 1651708800000
}
}
],
"link": [
{
"URL": "https://dx.plos.org/10.1371/journal.pone.0267462",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "340",
"original-title": [],
"page": "e0267462",
"prefix": "10.1371",
"published": {
"date-parts": [
[
2022,
5,
5
]
]
},
"published-online": {
"date-parts": [
[
2022,
5,
5
]
]
},
"publisher": "Public Library of Science (PLoS)",
"reference": [
{
"DOI": "10.1016/S2213-2600(20)30116-8",
"article-title": "Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?",
"author": "L Fang",
"doi-asserted-by": "crossref",
"first-page": "e21",
"journal-title": "Lancet Respir Med",
"key": "pone.0267462.ref001",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1136/annrheumdis-2020-217598",
"article-title": "Coronavirus disease 19 (Covid-19) and non-steroidal anti-inflammatory drugs (NSAID)",
"author": "A Giollo",
"doi-asserted-by": "crossref",
"first-page": "e12",
"journal-title": "Ann Rheum Dis",
"key": "pone.0267462.ref002",
"volume": "80",
"year": "2021"
},
{
"DOI": "10.1016/j.joca.2013.06.026",
"article-title": "The effects of nonsteroidal anti-inflammatory drugs on clinical outcomes, synovial fluid cytokine concentration and signal transduction pathways in knee osteoarthritis. A randomized open label trial",
"author": "L Gallelli",
"doi-asserted-by": "crossref",
"first-page": "1400",
"journal-title": "Osteoarthritis Cartilage",
"key": "pone.0267462.ref003",
"volume": "21",
"year": "2013"
},
{
"DOI": "10.1016/j.jcf.2015.03.007",
"article-title": "Use of ibuprofen to assess inflammatory biomarkers in induced sputum: Implications for clinical trials in cystic fibrosis",
"author": "JF Chmiel",
"doi-asserted-by": "crossref",
"first-page": "720",
"journal-title": "J Cyst Fibros",
"key": "pone.0267462.ref004",
"volume": "14",
"year": "2015"
},
{
"DOI": "10.1136/dtb.2020.000021",
"article-title": "EMA advice on the use of NSAIDs for Covid-19",
"doi-asserted-by": "crossref",
"first-page": "69",
"journal-title": "Drug Ther Bull",
"key": "pone.0267462.ref005",
"volume": "58",
"year": "2020"
},
{
"author": "United States Food and Drug Administration",
"key": "pone.0267462.ref006",
"volume-title": "Drug Safety and Availability [Internet]"
},
{
"author": "United States Department of Health and Human Services",
"key": "pone.0267462.ref007",
"volume-title": "COVID-19 [Internet].",
"year": "2020"
},
{
"author": "United States Department of Health and Human Services",
"key": "pone.0267462.ref008",
"volume-title": "COVID-19 [Internet]."
},
{
"DOI": "10.1016/S0140-6736(13)60900-9",
"article-title": "Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials",
"author": "Coxib and traditional NSAID Trialists’ (CNT) Collaboration",
"doi-asserted-by": "crossref",
"first-page": "769",
"journal-title": "Lancet",
"key": "pone.0267462.ref009",
"volume": "382",
"year": "2013"
},
{
"DOI": "10.1093/eurheartj/ehl053",
"article-title": "NSAID use and the risk of hospitalization for first myocardial infarction in the general population: a nationwide case-control study from Finland",
"author": "A Helin-Salmivaara",
"doi-asserted-by": "crossref",
"first-page": "1657",
"journal-title": "Eur Heart J",
"key": "pone.0267462.ref010",
"volume": "27",
"year": "2006"
},
{
"DOI": "10.1136/bmj.d3450",
"article-title": "Non-steroidal anti-inflammatory drug use and risk of atrial fibrillation or flutter: population based case-control study",
"author": "M Schmidt",
"doi-asserted-by": "crossref",
"first-page": "d3450",
"journal-title": "BMJ",
"key": "pone.0267462.ref011",
"volume": "343",
"year": "2011"
},
{
"DOI": "10.1161/CIRCULATIONAHA.105.602425",
"article-title": "Use of first- and second-generation cyclooxygenase-2-selective nonsteroidal antiinflammatory drugs and risk of acute myocardial infarction",
"author": "F Andersohn",
"doi-asserted-by": "crossref",
"first-page": "1950",
"journal-title": "Circulation",
"key": "pone.0267462.ref012",
"volume": "113",
"year": "2006"
},
{
"DOI": "10.1161/CIRCULATIONAHA.105.595793",
"article-title": "Nonsteroidal antiinflammatory drugs, acetaminophen, and the risk of cardiovascular events",
"author": "AT Chan",
"doi-asserted-by": "crossref",
"first-page": "1578",
"journal-title": "Circulation",
"key": "pone.0267462.ref013",
"volume": "113",
"year": "2006"
},
{
"author": "N. Arnold",
"first-page": "1",
"key": "pone.0267462.ref014",
"year": "2019"
},
{
"DOI": "10.1186/s12874-017-0338-0",
"article-title": "Double-adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance.",
"author": "TL Nguyen",
"doi-asserted-by": "crossref",
"first-page": "78",
"journal-title": "BMC Med Res Methodol",
"key": "pone.0267462.ref015",
"volume": "17",
"year": "2017"
},
{
"DOI": "10.1097/MLR.0000000000000325",
"article-title": "Estimating subgroup effects using the propensity score method: a practical application in outcomes research",
"author": "HV Eeren",
"doi-asserted-by": "crossref",
"first-page": "366",
"journal-title": "Med Care",
"key": "pone.0267462.ref016",
"volume": "53",
"year": "2015"
},
{
"DOI": "10.1002/sim.2580",
"article-title": "A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study",
"author": "PC Austin",
"doi-asserted-by": "crossref",
"first-page": "734",
"journal-title": "Stat Med",
"key": "pone.0267462.ref017",
"volume": "26",
"year": "2007"
},
{
"DOI": "10.1177/0962280210386207",
"article-title": "Diagnosing and responding to violations in the positivity assumption",
"author": "ML Petersen",
"doi-asserted-by": "crossref",
"first-page": "31",
"journal-title": "Stat Methods Med Res",
"key": "pone.0267462.ref018",
"volume": "21",
"year": "2012"
},
{
"article-title": "Association between NSAIDs use and adverse clinical outcomes among adults hospitalized with COVID-19 in South Korea: A nationwide study",
"author": "HE Jeong",
"first-page": "e4179",
"journal-title": "Clin Infect Dis",
"key": "pone.0267462.ref019",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.3390/jcm9082586",
"article-title": "Prior routine use of non-steroidal anti-inflammatory drugs (NSAIDs) and important outcomes in hospitalised patients with COVID-19",
"author": "E Bruce",
"doi-asserted-by": "crossref",
"first-page": "2586",
"journal-title": "J Clin Med",
"key": "pone.0267462.ref020",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1002/art.41593",
"article-title": "Non-steroidal anti-inflammatory drugs and susceptibility to COVID-19",
"author": "JS Chandan",
"doi-asserted-by": "crossref",
"first-page": "731",
"journal-title": "Arthritis Rheumatol",
"key": "pone.0267462.ref021",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1371/journal.pmed.1003308",
"article-title": "Adverse outcomes and mortality in users of non-steroidal anti-inflammatory drugs who tested positive for SARS-CoV-2: A Danish nationwide cohort study",
"author": "LC Lund",
"doi-asserted-by": "crossref",
"first-page": "e1003308",
"journal-title": "PLoS Med",
"key": "pone.0267462.ref022",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1007/s40121-020-00363-w",
"article-title": "Ibuprofen and NSAID use in COVID-19 infected patients is not associated with worse outcomes: a prospective cohort study",
"author": "LC Abu Esba",
"doi-asserted-by": "crossref",
"first-page": "253",
"journal-title": "Infect Dis Ther",
"key": "pone.0267462.ref023",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1213/ANE.0000000000005292",
"article-title": "Aspirin use is associated with decreased mechanical ventilation, intensive care unit admission, and in-hospital mortality in hospitalized patients with Coronavirus Disease 2019",
"author": "JH Chow",
"doi-asserted-by": "crossref",
"first-page": "930",
"journal-title": "Anesth Analg",
"key": "pone.0267462.ref024",
"volume": "132",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2020.561674",
"article-title": "Celebrex adjuvant therapy on Coronavirus Disease 2019: an experimental study.",
"author": "W Hong",
"doi-asserted-by": "crossref",
"first-page": "561674",
"journal-title": "Front Pharmacol",
"key": "pone.0267462.ref025",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1111/cts.12904",
"article-title": "Association between prescribed ibuprofen and severe COVID-19 infection: a nationwide register-based cohort study.",
"author": "K Kragholm",
"doi-asserted-by": "crossref",
"first-page": "1103",
"journal-title": "Clin Transl Sci",
"key": "pone.0267462.ref026",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1002/ajh.26102",
"article-title": "Intermediate-dose anticoagulation, aspirin, and in-hospital mortality in COVID-19: A propensity score-matched analysis",
"author": "ML Meizlish",
"doi-asserted-by": "crossref",
"first-page": "471",
"journal-title": "Am J Hematol",
"key": "pone.0267462.ref027",
"volume": "96",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0246825",
"article-title": "Association of mortality and aspirin prescription for COVID-19 patients at the Veterans Health Administration.",
"author": "TF Osborne",
"doi-asserted-by": "crossref",
"first-page": "e0246825",
"journal-title": "PLoS One",
"key": "pone.0267462.ref028",
"volume": "16",
"year": "2021"
},
{
"article-title": "SARS-CoV-2 receptors are expressed on human platelets and the effect of aspirin on clinical outcomes in COVID-19 patients.",
"author": "A Sahai",
"journal-title": "Res Sq: rs-119031 [Preprint].",
"key": "pone.0267462.ref029",
"year": "2020"
},
{
"article-title": "Use of non-steroidal anti-inflammatory and risk of death from COVID-19: an OpenSAFELY cohort analysis based on two cohorts",
"author": "AYS Wong",
"first-page": "943",
"journal-title": "BMJ",
"key": "pone.0267462.ref030",
"volume": "80",
"year": "2021"
},
{
"DOI": "10.1016/j.dsx.2021.102319",
"article-title": "Efficacy of naproxen in the management of patients hospitalized with COVID-19 infection: a randomized, double-blind, placebo-controlled clinical trial",
"author": "M Asadi",
"doi-asserted-by": "crossref",
"first-page": "102319",
"journal-title": "Diabetes Metab Syndr",
"key": "pone.0267462.ref031",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1177/1358863X211012754",
"article-title": "Effects of aspirin on short-term outcomes in hospitalized patients with COVID-19.",
"author": "A Sahai",
"doi-asserted-by": "crossref",
"first-page": "626",
"journal-title": "Vasc Med.",
"key": "pone.0267462.ref032",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.1097/ALN.0000000000003999",
"article-title": "Treatments associated with lower mortality among critially ill COVID-19 patients: a retrospective cohort study",
"author": "X Zhao",
"doi-asserted-by": "crossref",
"first-page": "1076",
"journal-title": "Anesthesiology",
"key": "pone.0267462.ref033",
"volume": "135",
"year": "2021"
},
{
"DOI": "10.1097/MD.0000000000026670",
"article-title": "Effect of aspirin on coronavirus disease 2019: a nationwide case-control study in South Korea.",
"author": "M Son",
"doi-asserted-by": "crossref",
"first-page": "e26670",
"issue": "30",
"journal-title": "Medicine",
"key": "pone.0267462.ref034",
"volume": "100",
"year": "2021"
},
{
"DOI": "10.1002/art.41593",
"author": "JS Chandan",
"doi-asserted-by": "crossref",
"first-page": "731",
"journal-title": "Arthritis Rheumatol",
"key": "pone.0267462.ref035",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1161/CIRCULATIONAHA.110.963843",
"article-title": "Aspirin: a historical and contemporary therapeutic overview",
"author": "V Fuster",
"doi-asserted-by": "crossref",
"first-page": "768",
"journal-title": "Circulation",
"key": "pone.0267462.ref036",
"volume": "123",
"year": "2011"
},
{
"DOI": "10.1136/bmj.324.7329.71",
"article-title": "Collective meta-analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.",
"author": "Antithrombotic Trialists Collaboration",
"doi-asserted-by": "crossref",
"first-page": "71",
"journal-title": "BMJ",
"key": "pone.0267462.ref037",
"volume": "324",
"year": "2002"
},
{
"DOI": "10.1093/eurheartj/ehv505",
"article-title": "Cardiovascular safety of non-aspirin non-steroidal anti-inflammatory drugs: review and position paper by the working group for Cardiovascular Pharmacotherapy of the European Society of Cardiology",
"author": "M Schmidt",
"doi-asserted-by": "crossref",
"first-page": "1015",
"journal-title": "Eur Heart J",
"key": "pone.0267462.ref038",
"volume": "37",
"year": "2016"
},
{
"author": "PR Rosenbaum",
"edition": "2",
"first-page": "4267",
"key": "pone.0267462.ref039",
"volume-title": "Encyclopaedia of biostatistics.",
"year": "2005"
},
{
"author": "Department of Veterans Affairs Veterans Health Administration Office of Policy and Planning",
"key": "pone.0267462.ref040",
"volume-title": "Healthcare Services [Internet].",
"year": "2018"
},
{
"DOI": "10.1177/1077558715617382",
"article-title": "Do Veterans Health Administration enrollees generalize to other populations?",
"author": "ES Wong",
"doi-asserted-by": "crossref",
"first-page": "493",
"journal-title": "Med Care Res Rev",
"key": "pone.0267462.ref041",
"volume": "73",
"year": "2016"
}
],
"reference-count": 41,
"references-count": 41,
"relation": {},
"resource": {
"primary": {
"URL": "https://dx.plos.org/10.1371/journal.pone.0267462"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "Chronic use of non-steroidal anti-inflammatory drugs (NSAIDs) or acetaminophen and relationship with mortality among United States Veterans after testing positive for COVID-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1371/journal.pone.corrections_policy",
"volume": "17"
}
campbell2