Association Between Prescribed Ibuprofen and Severe COVID-19 Infection: A Nationwide Register-Based Cohort Study
et al., Clinical and Translational Science, doi:10.1111/cts.12904, Oct 2020
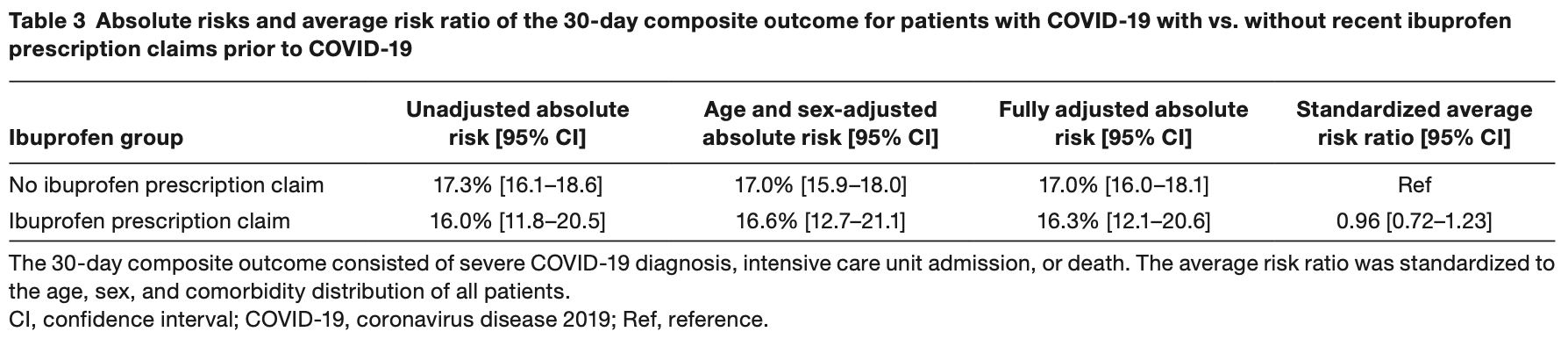
Retrospective 4,002 COVID-19 patients in Denmark, 264 with ibuprofen prescriptions, showing no significant difference for COVID-19 severity.
|
risk of progression, 4.0% lower, RR 0.96, p = 0.78, treatment 264, control 3,738.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kragholm et al., 21 Oct 2020, retrospective, Denmark, peer-reviewed, 13 authors, study period 1 January, 2020 - 30 April, 2020.
Contact: kdks@rn.dk.
Association Between Prescribed Ibuprofen and Severe COVID‐19 Infection: A Nationwide Register‐Based Cohort Study
Clinical and Translational Science, doi:10.1111/cts.12904
Recommendations regarding ibuprofen use in relation to coronavirus disease 2019 (COVID-19) have been conflicting. We examined the risk of severe COVID-19 between ibuprofen-prescribed and non-ibuprofen patients with COVID-19 in a nationwide register-based study of patients with COVID-19 in Denmark between the end of February 2020 and May 16, 2020. Patients with heart failure (n = 208), < 30 years (n = 575), and prescribed other nonsteroidal anti-inflammatory drugs (n = 57) were excluded. Patients with ibuprofen prescription claims between January 1, 2020, and before COVID-19 diagnosis or April 30, 2020 (last available prescription) were compared with patients without ibuprofen prescription claims. Outcome was a 30-day composite of severe COVID-19 diagnosis with acute respiratory syndrome, intensive care unit admission, or death. Absolute risks and average risk ratios comparing outcome for ibuprofen vs. non-ibuprofen patients standardized to the age, sex, and comorbidity distribution of all patients were derived from multivariable Cox regression. Among 4,002 patients, 264 (6.6%) had ibuprofen prescription claims before COVID-19. Age, sex, and comorbidities were comparable between the two study groups. Standardized absolute risks of the composite outcome for ibuprofen-prescribed vs. non-ibuprofen patients were 16.3% (95% confidence interval (CI) 12.1-20.6) vs. 17.0% (95% CI 16.0-18.1), P = 0.74. The standardized average risk ratio for ibuprofen-prescribed vs. non-ibuprofen patients was 0.96 (95% CI 0.72-1.23). Standardized absolute risks of the composite outcome for patients with ibuprofen prescription claims > 14 days before COVID-19 vs. ≤ 14 days of COVID-19 were 17.1% (95% CI 12.3-22.0) vs. 14.3% (95% CI 7.1-23.1). In conclusion, in this nationwide study, there was no significant association between ibuprofen prescription claims and severe COVID-19.
Conflict of Interest. The authors declared no competing interests for this work. Author Contributions. K.K. wrote the manuscript. K.K., C.T.P., E.F., G.G., M.S., and L.K. designed the research. K.K., T.A.G., E.F., M.P.A., M.P., J.H.B., J.P., C.B., L.Ø., and C.T.P. performed the research. K.K., T.A.G., and C.T.P. analyzed the data.
References
Boccia, Ricciardi, Ioannidis, What other countries can learn from Italy during the COVID-19 pandemic, JAMA Intern. Med
Capuano, Scavone, Racagni, Scaglione, Italian Society of Pharmacology. NSAIDs in patients with viral infections, including Covid-19: victims or perpetrators?, Pharmacol. Res
Core, R: A language and environment for statistical computing (R Foundation for Statistical Computing
Day, Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists, BMJ
Emanuel, Fair allocation of scarce medical resources in the time of Covid-19, N. Engl. J. Med
Fang, Karakiulakis, Roth, Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?, Lancet Respir. Med
Grant, Converting an odds ratio to a range of plausible relative risks for better communication of research findings, BMJ
Grasselli, Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy, JAMA
Kinross, Rapidly increasing cumulative incidence of coronavirus disease (COVID-19) in the European Union/European Economic Area and the United Kingdom, 1, Euro Surveill
Krogager, Short-term mortality risk of serum potassium levels in hypertension: a retrospective analysis of nationwide registry data, Eur. Heart J
Kuba, A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury, Nat. Med
Moore, Carleton, Blin, Bosco-Levy, Droz, Does Ibuprofen Worsen COVID-19?, Drug Saf
Petrilli, Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study, BMJ
Rinott, Kozer, Shapira, Bar-Haim, Youngster, Ibuprofen use and clinical outcomes in COVID-19 patients, Clin. Microbiol. Infect
Rubino, Kelvin, Bermejo-Martin, Kelvin, As COVID-19 cases, deaths and fatality rates surge in Italy, underlying causes require investigation, J. Infect. Dev. Ctries
Russell, Moss, Rigg, Van Hemelrijck, COVID-19 and treatment with NSAIDs and corticosteroids: should we be limiting their use in the clinical setting?, Ecancermedicalscience
Vaja, The COVID-19 ibuprofen controversy: a systematic review of NSAIDs in adult acute lower respiratory tract infections, Br. J. Clin. Pharmacol, doi:10.1111/bcp.14514
Zhu, A novel coronavirus from patients with pneumonia in China, N. Engl. J. Med
DOI record:
{
"DOI": "10.1111/cts.12904",
"ISSN": [
"1752-8054",
"1752-8062"
],
"URL": "http://dx.doi.org/10.1111/cts.12904",
"alternative-id": [
"10.1111/cts.12904"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2020-07-24"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2020-09-10"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2020-10-21"
}
],
"author": [
{
"affiliation": [
{
"name": "Unit of Clinical Biostatistics and Epidemiology Aalborg University Hospital Aalborg Denmark"
},
{
"name": "Departments of Cardiology North Denmark Regional Hospital and Aalborg University Hospital Aalborg Denmark"
}
],
"family": "Kragholm",
"given": "Kristian",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Biostatistics Copenhagen University Copenhagen Denmark"
}
],
"family": "Gerds",
"given": "Thomas A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiology Rigshospitalet Copenhagen Denmark"
}
],
"family": "Fosbøl",
"given": "Emil",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Research Nordsjaellands Hospital Hillerød Denmark"
}
],
"family": "Andersen",
"given": "Mikkel Porsborg",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The Danish Heart Foundation Copenhagen Denmark"
}
],
"family": "Phelps",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiology Rigshospitalet Copenhagen Denmark"
}
],
"family": "Butt",
"given": "Jawad H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiology Rigshospitalet Copenhagen Denmark"
}
],
"family": "Østergaard",
"given": "Lauge",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Research Nordsjaellands Hospital Hillerød Denmark"
},
{
"name": "The Danish Heart Foundation Copenhagen Denmark"
}
],
"family": "Bang",
"given": "Casper N.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiology Herlev‐Gentofte Hospital Copenhagen Denmark"
}
],
"family": "Pallisgaard",
"given": "Jannik",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The Danish Heart Foundation Copenhagen Denmark"
},
{
"name": "Department of Cardiology Herlev‐Gentofte Hospital Copenhagen Denmark"
}
],
"family": "Gislason",
"given": "Gunnar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiology Herlev‐Gentofte Hospital Copenhagen Denmark"
}
],
"family": "Schou",
"given": "Morten",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiology Rigshospitalet Copenhagen Denmark"
}
],
"family": "Køber",
"given": "Lars",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Research Nordsjaellands Hospital Hillerød Denmark"
}
],
"family": "Torp‐Pedersen",
"given": "Christian",
"sequence": "additional"
}
],
"container-title": "Clinical and Translational Science",
"container-title-short": "Clin Transl Sci",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2020,
9,
24
]
],
"date-time": "2020-09-24T18:44:40Z",
"timestamp": 1600973080000
},
"deposited": {
"date-parts": [
[
2020,
12,
6
]
],
"date-time": "2020-12-06T18:22:17Z",
"timestamp": 1607278937000
},
"indexed": {
"date-parts": [
[
2022,
7,
18
]
],
"date-time": "2022-07-18T09:28:26Z",
"timestamp": 1658136506648
},
"is-referenced-by-count": 18,
"issue": "6",
"issued": {
"date-parts": [
[
2020,
10,
21
]
]
},
"journal-issue": {
"issue": "6",
"published-print": {
"date-parts": [
[
2020,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
10,
21
]
],
"date-time": "2020-10-21T00:00:00Z",
"timestamp": 1603238400000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
10,
21
]
],
"date-time": "2020-10-21T00:00:00Z",
"timestamp": 1603238400000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/cts.12904",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/cts.12904",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/cts.12904",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "1103-1107",
"prefix": "10.1111",
"published": {
"date-parts": [
[
2020,
10,
21
]
]
},
"published-online": {
"date-parts": [
[
2020,
10,
21
]
]
},
"published-print": {
"date-parts": [
[
2020,
11
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1136/bmj.m1966",
"article-title": "Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study",
"author": "Petrilli C.M.",
"doi-asserted-by": "crossref",
"first-page": "m1966",
"journal-title": "BMJ",
"key": "e_1_2_10_1_1",
"volume": "22",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001017",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_2_1"
},
{
"DOI": "10.1056/NEJMsb2005114",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_3_1"
},
{
"DOI": "10.2807/1560-7917.ES.2020.25.11.2000285",
"article-title": "Rapidly increasing cumulative incidence of coronavirus disease (COVID‐19) in the European Union/European Economic Area and the United Kingdom, 1 January to 15 March 2020",
"author": "Kinross P.",
"doi-asserted-by": "crossref",
"first-page": "2000285",
"journal-title": "Euro Surveill.",
"key": "e_1_2_10_4_1",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1001/jamainternmed.2020.1447",
"article-title": "What other countries can learn from Italy during the COVID‐19 pandemic",
"author": "Boccia S.",
"doi-asserted-by": "crossref",
"first-page": "927",
"journal-title": "JAMA Intern. Med.",
"key": "e_1_2_10_5_1",
"volume": "180",
"year": "2020"
},
{
"DOI": "10.3855/jidc.12734",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_6_1"
},
{
"DOI": "10.1001/jama.2020.5394",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_7_1"
},
{
"DOI": "10.1038/nm1267",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_8_1"
},
{
"DOI": "10.1016/S2213-2600(20)30116-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_9_1"
},
{
"DOI": "10.3332/ecancer.2020.1023",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_10_1"
},
{
"key": "e_1_2_10_11_1",
"unstructured": "The Danish Health Data Authority.Management of COVID‐19 in healthcare cost and financing<https://sundhedsdatastyrelsen.dk/‐/media/sds/filer/finansiering‐og‐afregning/gruppering/noegler/2020/bilag‐2‐_‐haandtering‐af‐covid_19‐i‐drg.pdf>. Accessed on August 31 2020."
},
{
"article-title": "Short‐term mortality risk of serum potassium levels in hypertension: a retrospective analysis of nationwide registry data",
"author": "Krogager M.L.",
"first-page": "104",
"journal-title": "Eur. Heart J.",
"key": "e_1_2_10_12_1",
"volume": "38",
"year": "2017"
},
{
"DOI": "10.1136/bmj.f7450",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_13_1"
},
{
"author": "R Core Team",
"key": "e_1_2_10_14_1",
"volume-title": "R: A language and environment for statistical computing",
"year": "2017"
},
{
"DOI": "10.1136/bmj.m1086",
"article-title": "Covid‐19: ibuprofen should not be used for managing symptoms, say doctors and scientists",
"author": "Day M.",
"doi-asserted-by": "crossref",
"first-page": "m1086",
"journal-title": "BMJ",
"key": "e_1_2_10_15_1",
"volume": "17",
"year": "2020"
},
{
"article-title": "The COVID‐19 ibuprofen controversy: a systematic review of NSAIDs in adult acute lower respiratory tract infections",
"author": "Vaja R.",
"journal-title": "Br. J. Clin. Pharmacol.",
"key": "e_1_2_10_16_1",
"year": "2020"
},
{
"DOI": "10.1016/j.cmi.2020.06.003",
"article-title": "Ibuprofen use and clinical outcomes in COVID‐19 patients",
"author": "Rinott E.",
"doi-asserted-by": "crossref",
"first-page": "1259.e5",
"journal-title": "Clin. Microbiol. Infect.",
"key": "e_1_2_10_17_1",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1007/s40264-020-00953-0",
"article-title": "Does Ibuprofen Worsen COVID‐19?",
"author": "Nicholas Moore N.",
"doi-asserted-by": "crossref",
"first-page": "611",
"journal-title": "Drug Saf.",
"key": "e_1_2_10_18_1",
"volume": "43",
"year": "2020"
},
{
"DOI": "10.1016/j.phrs.2020.104849",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_19_1"
}
],
"reference-count": 19,
"references-count": 19,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1111/cts.12904"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Pharmacology, Toxicology and Pharmaceutics",
"General Biochemistry, Genetics and Molecular Biology",
"General Medicine",
"General Neuroscience"
],
"subtitle": [],
"title": "Association Between Prescribed Ibuprofen and Severe COVID‐19 Infection: A Nationwide Register‐Based Cohort Study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "13"
}