Nonsteroidal Antiinflammatory Drugs and Susceptibility to COVID-19
et al., Arthritis & Rheumatology, doi:10.1002/art.41593, Apr 2021
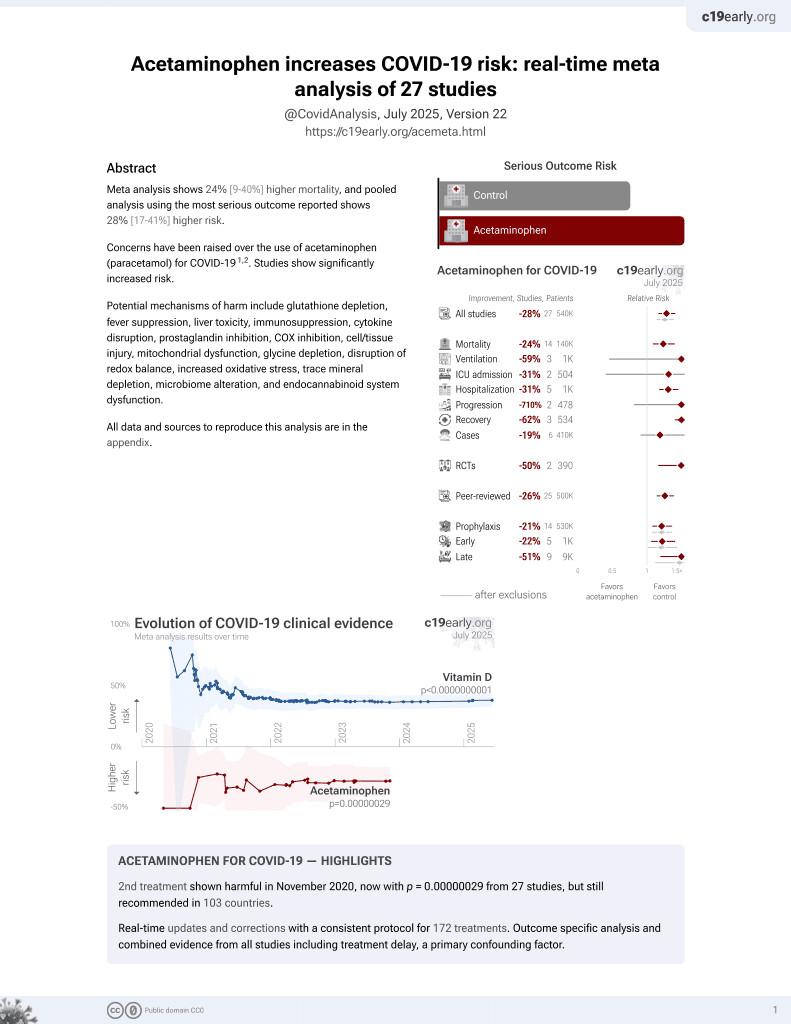
2nd treatment shown to increase risk in
November 2020, now with p = 0.00000029 from 27 studies, but still recommended in 103 countries.
6,400+ studies for
210+ treatments. c19early.org
|
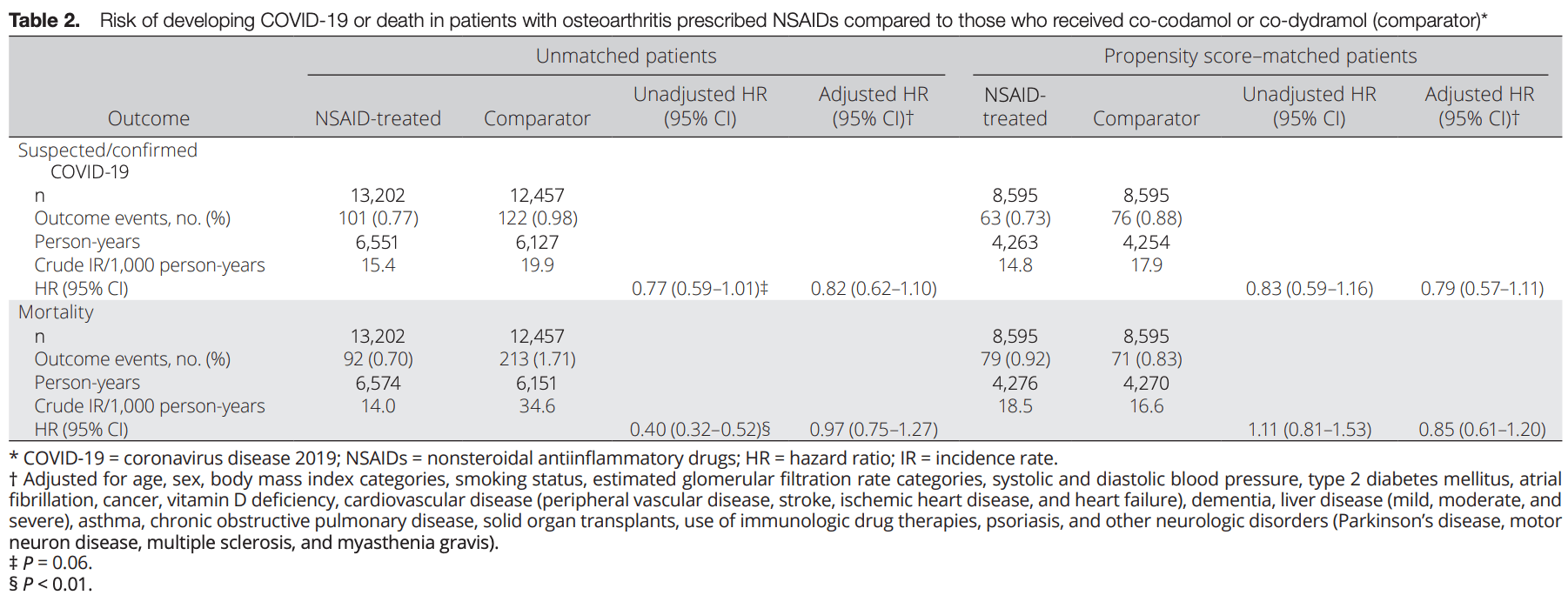
Retrospective 12,457 patients prescribed paracetamol with codeine/dihydrocodeine and 13,202 prescribed NSAIDs, showing no significant differences in cases and mortality. Patients prescribed codeine/dihydrocodeine may have different susceptibility to COVID-19.
Paracetamol is also known as acetaminophen, Tylenol, Panadol, Calpol, Tempra, Calprofen, Doliprane, Efferalgan, Grippostad C, Dolo, Acamol, Fevadol, Crocin, and Perfalgan.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments1.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 17.6% higher, HR 1.18, p = 0.35, treatment 71 of 8,595 (0.8%), control 79 of 8,595 (0.9%), adjusted per study, inverted to make HR<1 favor treatment, propensity score matching, multivariable.
|
|
risk of case, 26.6% higher, HR 1.27, p = 0.17, treatment 8,595, control 8,595, adjusted per study, inverted to make HR<1 favor treatment, propensity score matching, multivariable.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Chandan et al., 29 Apr 2021, retrospective, United Kingdom, peer-reviewed, mean age 65.4, 24 authors, study period 30 January, 2020 - 31 July, 2020, this trial compares with another treatment - results may be better when compared to placebo, this trial uses multiple treatments in the treatment arm (combined with codeine or dihydrocodeine) - results of individual treatments may vary.
Contact: k.nirantharan@bham.ac.uk, k.raza@bham.ac.uk.
Nonsteroidal Antiinflammatory Drugs and Susceptibility to COVID‐19
Arthritis & Rheumatology, doi:10.1002/art.41593
Objective. To identify whether active use of nonsteroidal antiinflammatory drugs (NSAIDs) increases susceptibility to developing suspected or confirmed coronavirus disease 2019 (COVID-19) compared to the use of other common analgesics. Methods. We performed a propensity score-matched cohort study with active comparators, using a large UK primary care data set. The cohort consisted of adult patients age ≥18 years with osteoarthritis (OA) who were followed up from January 30 to July 31, 2020. Patients prescribed an NSAID (excluding topical preparations) were compared to those prescribed either co-codamol (paracetamol and codeine) or co-dydramol (paracetamol and dihydrocodeine). A total of 13,202 patients prescribed NSAIDs were identified, compared to 12,457 patients prescribed the comparator drugs. The primary outcome measure was the documentation of suspected or confirmed COVID-19, and the secondary outcome measure was all-cause mortality. Results. During follow-up, the incidence rates of suspected/confirmed COVID-19 were 15.4 and 19.9 per 1,000 person-years in the NSAID-exposed group and comparator group, respectively. Adjusted hazard ratios for suspected or confirmed COVID-19 among the unmatched and propensity score-matched OA cohorts, using data from clinical consultations in primary care settings, were 0.82 (95% confidence interval [95% CI] 0.62-1.10) and 0.79 (95% CI 0.57-1.11), respectively, and adjusted hazard ratios for the risk of all-cause mortality were 0.97 (95% CI 0.75-1.27) and 0.85 (95% CI 0.61-1.20), respectively. There was no effect modification by age or sex.
Conclusion. No increase in the risk of suspected or confirmed COVID-19 or mortality was observed among patients with OA in a primary care setting who were prescribed NSAIDs as compared to those who received comparator drugs. These results are reassuring and suggest that in the absence of acute illness, NSAIDs can be safely prescribed during the ongoing pandemic.
ADDITIONAL DISCLOSURES Authors Byne, Dhalla, and Acosta-Mena are employees of Cegedim Rx.
References
Baigent, Bhala, Emberson, Merhi, Abramson et al., Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials, Lancet
Basille, Thomsen, Madsen, Duhaut, Andrejak et al., Nonsteroidal antiinflammatory drug use and clinical outcomes of community-acquired pneumonia, Am J Respir Crit Care Med
Blak, Thompson, Dattani, Bourke, Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates, Inform Prim Care
Booth, What are the Read Codes?, Health Libr Rev
Bourgeois, Ferroni, Leruez-Ville, Varon, Thumerelle et al., Nonsteroidal anti-inflammatory drug without antibiotics for acute viral infection increases the empyema risk in children: a matched case-control study, J Pediatr
Collaborative, Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans, Br J Surg
Davis, Lee, Kim, Advani, Peng et al., Use of non-steroidal anti-inflammatory drugs in US adults: changes over time and by demographic, Open Hear
Day, Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists, BMJ
Dubreuil, Louie-Gao, Peloquin, Choi, Zhang et al., Risk of myocardial infarction with use of selected non-steroidal anti-inflammatory drugs in patients with spondyloarthritis and osteoarthritis, Ann Rheum Dis
Edelman, Gordon, Crothers, Akgün, Bryant et al., Association of prescribed opioids with increased risk of community-acquired pneumonia among patients with and without HIV, JAMA Intern Med
Fang, Karakiulakis, Roth, Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?
Gokhale, Chandan, Toulis, Gkoutos, Tino et al., Data extraction for epidemiological research (DExtER): a novel tool for automated clinical epidemiology studies, Eur J Epidemiol
Haroon, Subramanian, Cooper, Anand, Gokhale et al., Renin-angiotensin system inhibitors and susceptibility to COVID-19 in patients with hypertension: a propensity score-matched cohort study in primary care, BMC Infect Dis
Health Organization, The use of non-steroidal antiinflammatory drugs (NSAIDs) in patients with COVID-19
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell
Hopkins, COVID-19 Cashboard by the Center for Systems Science and Engineering
Jeong, Lee, Shin, Choe, Filion et al., Association between NSAIDs use and adverse clinical outcomes among adults hospitalised with COVID-19 in South Korea: a nationwide study, Clin Infect Dis
Little, Non-steroidal anti-inflammatory drugs and COVID-19 [editorial, BMJ
Lund, Kristensen, Reilev, Christensen, Thomsen et al., Adverse outcomes and mortality in users of non-steroidal anti-inflammatory drugs tested positive for SARS-CoV-2: a Danish nationwide cohort study, PLoS Med
Maguire, Blak, Thompson, The importance of defining periods of complete mortality reporting for research using automated data from primary care, Pharmacoepidemiol Drug Saf
Nhs England, Acute use of non-steroidal anti-inflammatory drugs (NSAIDs) in people with or at risk of COVID-19
Qiao, Wang, Chen, Zhang, Liu et al., Ibuprofen attenuates cardiac fibrosis in streptozotocin-induced diabetic rats, Cardiology
Sainsbury, Wang, Gokhale, Acosta-Mena, Dhalla et al., Sodium-glucose co-transporter-2 inhibitors and susceptibility to COVID-19: a population-based retrospective cohort study, Diabetes Obes Metab
Sungnak, Huang, Bécavin, Berg, Queen et al., SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes, Nat Med
Voiriot, Philippot, Elabbadi, Elbim, Chalumeau et al., Risks related to the use of non-steroidal anti-inflammatory drugs in community-acquired pneumonia in adult and pediatric patients
Wei, Neogi, Terkeltaub, Fenves, Zeng et al., Thiazide diuretics and risk of knee replacement surgery among patients with knee osteoarthritis: a general population-based cohort study, Osteoarthritis Cartilage
Wei, Wood, Dubreuil, Tomasson, Larochelle et al., Association of tramadol with risk of myocardial infarction among patients with osteoarthritis, Osteoarthritis Cartilage
Williamson, Walker, Bhaskaran, Bacon, Bates et al., Factors associated with COVID-19 death using OpenSAFELY, Nature
Zeng, Dubreuil, Larochelle, Lu, Wei et al., Association of tramadol with all-cause mortality among patients with osteoarthritis, JAMA
Zhou, Lou, Wang, Hu, Zhang et al., A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature
DOI record:
{
"DOI": "10.1002/art.41593",
"ISSN": [
"2326-5191",
"2326-5205"
],
"URL": "http://dx.doi.org/10.1002/art.41593",
"alternative-id": [
"10.1002/art.41593"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2020-09-13"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2020-11-10"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2021-04-29"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-9561-5141",
"affiliation": [
{
"name": "Institute of Applied Health Research University of Birmingham, Birmingham, UK, and Warwick Medical School University of Warwick Coventry UK"
}
],
"authenticated-orcid": false,
"family": "Chandan",
"given": "Joht Singh",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Institute of Applied Health Research University of Birmingham Birmingham UK"
}
],
"family": "Zemedikun",
"given": "Dawit Tefra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Applied Health Research University of Birmingham Birmingham UK"
}
],
"family": "Thayakaran",
"given": "Rasiah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Cegedim Rx London UK"
}
],
"family": "Byne",
"given": "Nathan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Cegedim Rx London UK"
}
],
"family": "Dhalla",
"given": "Samir",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Cegedim Rx London UK"
}
],
"family": "Acosta‐Mena",
"given": "Dionisio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Applied Health Research University of Birmingham Birmingham UK"
}
],
"family": "Gokhale",
"given": "Krishna M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2188-3784",
"affiliation": [
{
"name": "Kennedy Institute of Rheumatology University of Oxford Oxford UK"
}
],
"authenticated-orcid": false,
"family": "Thomas",
"given": "Tom",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Applied Health Research University of Birmingham Birmingham UK"
}
],
"family": "Sainsbury",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Applied Health Research University of Birmingham Birmingham UK"
}
],
"family": "Subramanian",
"given": "Anuradhaa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Applied Health Research University of Birmingham Birmingham UK"
}
],
"family": "Cooper",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Applied Health Research University of Birmingham Birmingham UK"
}
],
"family": "Anand",
"given": "Astha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Applied Health Research University of Birmingham Birmingham UK"
}
],
"family": "Okoth",
"given": "Kelvin O.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Applied Health Research University of Birmingham Birmingham UK"
}
],
"family": "Wang",
"given": "Jingya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Applied Health Research University of Birmingham Birmingham UK"
}
],
"family": "Adderley",
"given": "Nicola J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Applied Health Research University of Birmingham Birmingham UK"
}
],
"family": "Taverner",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Inflammation and Ageing University of Birmingham Queen Elizabeth Hospital Birmingham University Hospitals Birmingham NHS Foundation Trust Birmingham UK"
}
],
"family": "Denniston",
"given": "Alastair K.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Inflammation and Ageing University of Birmingham MRC Versus Arthritis Centre for Musculoskeletal Ageing Research University of Birmingham Birmingham UK"
}
],
"family": "Lord",
"given": "Janet",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Applied Health Research University of Birmingham Birmingham UK"
}
],
"family": "Thomas",
"given": "G. Neil",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Kennedy Institute of Rheumatology University of Oxford, Oxford, UK, and Institute of Inflammation and Ageing MRC Versus Arthritis Centre for Musculoskeletal Ageing Research University of Birmingham Birmingham UK"
}
],
"family": "Buckley",
"given": "Christopher D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Inflammation and Ageing MRC Versus Arthritis Centre for Musculoskeletal Ageing Research, Sandwell and West Birmingham NHS Hospitals Trust Birmingham UK"
}
],
"family": "Raza",
"given": "Karim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Applied Health Research University of Birmingham Queen Elizabeth Hospital Birmingham University Hospitals Birmingham NHS Foundation Trust Birmingham UK"
}
],
"family": "Bhala",
"given": "Neeraj",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Applied Health Research University of Birmingham Health Data Research UK Midlands Birmingham UK"
}
],
"family": "Nirantharakumar",
"given": "Krishnarajah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Applied Health Research University of Birmingham Birmingham UK"
}
],
"family": "Haroon",
"given": "Shamil",
"sequence": "additional"
}
],
"container-title": "Arthritis & Rheumatology",
"container-title-short": "Arthritis Rheumatol",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2020,
11,
13
]
],
"date-time": "2020-11-13T10:17:09Z",
"timestamp": 1605262629000
},
"deposited": {
"date-parts": [
[
2021,
5,
15
]
],
"date-time": "2021-05-15T11:56:50Z",
"timestamp": 1621079810000
},
"indexed": {
"date-parts": [
[
2022,
8,
26
]
],
"date-time": "2022-08-26T15:50:13Z",
"timestamp": 1661529013033
},
"is-referenced-by-count": 18,
"issue": "5",
"issued": {
"date-parts": [
[
2021,
4,
29
]
]
},
"journal-issue": {
"issue": "5",
"published-print": {
"date-parts": [
[
2021,
5
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
4,
29
]
],
"date-time": "2021-04-29T00:00:00Z",
"timestamp": 1619654400000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
4,
29
]
],
"date-time": "2021-04-29T00:00:00Z",
"timestamp": 1619654400000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/art.41593",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/art.41593",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/art.41593",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "731-739",
"prefix": "10.1002",
"published": {
"date-parts": [
[
2021,
4,
29
]
]
},
"published-online": {
"date-parts": [
[
2021,
4,
29
]
]
},
"published-print": {
"date-parts": [
[
2021,
5
]
]
},
"publisher": "Wiley",
"reference": [
{
"key": "e_1_2_7_2_1",
"unstructured": "World Health Organization.Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019‐nCoV). January2020. URL:https://www.who.int/news‐room/detail/30‐01‐2020‐statement‐on‐the‐second‐meeting‐of‐the‐international‐health‐regulations‐(2005)‐emergency‐committee‐regarding‐the‐outbreak‐of‐novel‐coronavirus‐(2019‐ncov)."
},
{
"key": "e_1_2_7_3_1",
"unstructured": "John Hopkins University.COVID‐19 Cashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). URL:https://coronavirus.jhu.edu/map.html."
},
{
"DOI": "10.1164/rccm.201802-0229LE",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_4_1"
},
{
"DOI": "10.1016/j.jpeds.2016.05.025",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_5_1"
},
{
"DOI": "10.3390/jcm8060786",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_6_1"
},
{
"DOI": "10.1136/bmj.m1086",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_7_1"
},
{
"DOI": "10.1136/bmj.m1185",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_8_1"
},
{
"DOI": "10.1038/s41586-020-2012-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_9_1"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_10_1"
},
{
"DOI": "10.1038/s41591-020-0868-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_11_1"
},
{
"DOI": "10.1016/S2213-2600(20)30116-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_12_1"
},
{
"DOI": "10.1159/000375362",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_13_1"
},
{
"article-title": "Elective surgery cancellations due to the COVID‐19 pandemic: global predictive modelling to inform surgical recovery plans",
"author": "COVIDSurg Collaborative",
"first-page": "1440",
"journal-title": "Br J Surg",
"key": "e_1_2_7_14_1",
"volume": "107",
"year": "2020"
},
{
"key": "e_1_2_7_15_1",
"unstructured": "NHS England.Acute use of non‐steroidal anti‐inflammatory drugs (NSAIDs) in people with or at risk of COVID‐19. April2020. URL:https://www.england.nhs.uk/coronavirus/publication/acute‐use‐of‐non‐steroidal‐anti‐inflammatory‐drugs/."
},
{
"DOI": "10.15557/PiMR.2020.0022",
"doi-asserted-by": "crossref",
"key": "e_1_2_7_16_1",
"unstructured": "World Health Organization.The use of non‐steroidal anti‐inflammatory drugs (NSAIDs) in patients with COVID‐19. April2020. URL:https://www.who.int/publications/i/item/the‐use‐of‐non‐steroidal‐anti‐inflammatory‐drugs‐(nsaids)‐in‐patients‐with‐covid‐19."
},
{
"article-title": "Association between NSAIDs use and adverse clinical outcomes among adults hospitalised with COVID‐19 in South Korea: a nationwide study",
"author": "Jeong HE",
"journal-title": "Clin Infect Dis",
"key": "e_1_2_7_17_1",
"year": "2020"
},
{
"DOI": "10.1371/journal.pmed.1003308",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_18_1"
},
{
"key": "e_1_2_7_19_1",
"unstructured": "King’s College London sponsor.LIBERATE Trial in COVID‐19 (LIBERATE). ClinicalTrials.gov identifier: NCT04334629;2020."
},
{
"article-title": "Data extraction for epidemiological research (DExtER): a novel tool for automated clinical epidemiology studies",
"author": "Gokhale KM",
"journal-title": "Eur J Epidemiol",
"key": "e_1_2_7_20_1",
"year": "2020"
},
{
"key": "e_1_2_7_21_1",
"unstructured": "THIN: The Health Improvement Network. URL:https://www.the‐health‐improvement‐network.com/en/."
},
{
"article-title": "Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates",
"author": "Blak BT",
"first-page": "251",
"journal-title": "Inform Prim Care",
"key": "e_1_2_7_22_1",
"volume": "19",
"year": "2011"
},
{
"DOI": "10.1001/jama.2019.1347",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_23_1"
},
{
"article-title": "Risk of myocardial infarction with use of selected non‐steroidal anti‐inflammatory drugs in patients with spondyloarthritis and osteoarthritis",
"author": "Dubreuil M",
"first-page": "1137",
"journal-title": "Ann Rheum Dis",
"key": "e_1_2_7_24_1",
"volume": "77",
"year": "2018"
},
{
"DOI": "10.1016/j.joca.2019.05.020",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_25_1"
},
{
"DOI": "10.1016/j.joca.2019.10.001",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_26_1"
},
{
"DOI": "10.1111/dom.14203",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_27_1"
},
{
"DOI": "10.1186/s12879-021-05951-w",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_28_1"
},
{
"DOI": "10.1046/j.1365-2532.1994.1130177.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_29_1"
},
{
"key": "e_1_2_7_30_1",
"unstructured": "World Health Organization.Anatomical Therapeutic Chemical (ATC) classification. URL:https://www.who.int/toolkits/atc‐ddd‐toolkit/atc‐classification."
},
{
"key": "e_1_2_7_31_1",
"unstructured": "National Health Service Business Services Authority.Dictionary of medicines and devices (dm+d). URL:https://www.nhsbsa.nhs.uk/pharmacies‐gp‐practices‐and‐appliance‐contractors/dictionary‐medicines‐and‐devices‐dmd."
},
{
"DOI": "10.1002/pds.1688",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_32_1"
},
{
"key": "e_1_2_7_33_1",
"unstructured": "OpenPrescribing.10.1.1: Non‐steroidal anti‐inflammatory drugs. URL:https://openprescribing.net/bnf/100101/."
},
{
"DOI": "10.1001/jamainternmed.2018.6101",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_34_1"
},
{
"DOI": "10.1038/s41586-020-2521-4",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_35_1"
},
{
"DOI": "10.1136/openhrt-2016-000550",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_36_1"
},
{
"DOI": "10.1016/S0140-6736(13)60900-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_37_1"
}
],
"reference-count": 36,
"references-count": 36,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/art.41593"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Immunology",
"Rheumatology",
"Immunology and Allergy"
],
"subtitle": [],
"title": "Nonsteroidal Antiinflammatory Drugs and Susceptibility to COVID‐19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "73"
}