Effect of aspirin on coronavirus disease 2019
et al., Medicine, doi:10.1097/MD.0000000000026670, Jul 2021
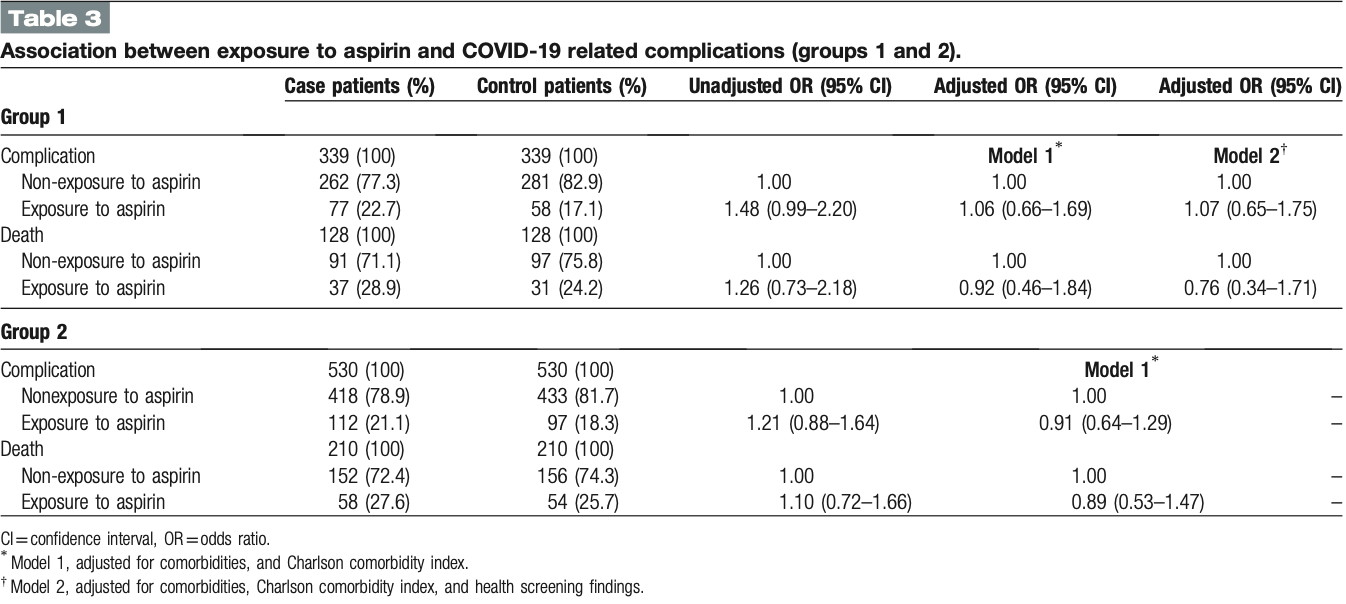
PSM retrospective case control study in South Korea, showing a trend towards lower mortality, but no significant differences with aspirin use.
|
risk of death, 11.0% lower, OR 0.89, p = 0.67, treatment 58 of 210 (27.6%) cases,
54 of 210 (25.7%) controls, adjusted per study, case control OR, group 2, model 1, multivariable.
|
|
risk of death, 24.0% lower, OR 0.76, p = 0.52, treatment 37 of 128 (28.9%) cases,
31 of 128 (24.2%) controls, adjusted per study, case control OR, group 1, model 2, multivariable.
|
|
risk of progression, 7.0% higher, OR 1.07, p = 0.80, treatment 77 of 339 (22.7%) cases,
58 of 339 (17.1%) controls, adjusted per study, case control OR, complications, group 1, model 2, multivariable.
|
|
risk of progression, 9.0% lower, OR 0.91, p = 0.61, treatment 77 of 339 (22.7%) cases,
58 of 339 (17.1%) controls, adjusted per study, case control OR, complications, group 2, model 1, multivariable.
|
|
risk of case, 11.0% higher, OR 1.11, p = 0.21, treatment 313 of 3,825 (8.2%) cases,
531 of 7,650 (6.9%) controls, adjusted per study, case control OR, group 1, PSM 1, model 2, multivariable.
|
|
risk of case, 1.0% higher, OR 1.01, p = 0.90, treatment 431 of 7,223 (6.0%) cases,
752 of 14,446 (5.2%) controls, adjusted per study, case control OR, group 2, PSM 1, model 1, multivariable.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Son et al., 30 Jul 2021, retrospective, propensity score matching, South Korea, peer-reviewed, 6 authors.
Effect of aspirin on coronavirus disease 2019
Medicine, doi:10.1097/md.0000000000026670
Several studies reported that aspirin can potentially help prevent infection and serious complications of coronavirus disease (COVID-19), but no study has elucidated a definitive association between aspirin and COVID-19. This study aims to investigate the association between aspirin and COVID-19. This case-control study used demographic, clinical, and health screening laboratory test data collected from the National Health Insurance Service database. Patients who tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection until June 4, 2020, were matched with control patients using propensity score matching according to their SARS-CoV-2 status, the composite of complications, and death. The composite of complications included intensive care unit admission, use of vasopressors, high-flow oxygen therapy, renal replacement therapy, extracorporeal membrane oxygenation, and death. Exposure to aspirin was defined as having a prescription for aspirin for more than 14 days, including the index date. After matching, multivariableadjusted conditional logistic regression analysis was performed. To confirm the robustness of this study, we used 2 study groups, 3 propensity score matching methods, and 3 models for conditional logistic regression analyses. The crude odds ratio and 95% confidence interval for SARS-CoV-2 infection between the groups without and with exposure to aspirin were 1.21 (1.04-1.41), but the adjusted odds ratios (95% confidence interval) were not significant. There was no association between aspirin exposure and COVID-19 status. Multiple statistical analyses, including subgroup analysis, revealed consistent results. Furthermore, the results of analysis for complications and death were not significant. Aspirin exposure was not associated with COVID-19-related complications and mortality in COVID-19 patients. In this nationwide population-based case-control study, aspirin use was not associated with SARS-CoV-2 infection or related complications. With several ongoing randomized controlled trials of aspirin in COVID-19 patients, more studies would be able to confirm the effectiveness of aspirin in COVID-19.
Author contributions
References
Alhazzani, Møller, Arabi, Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19), Intensive Care Med
Aly, Ibrahim, Should aspirin be used for prophylaxis of COVID-19-induced coagulopathy?, Med Hypotheses
Austin, An Introduction to propensity score methods for reducing the effects of confounding in observational studies, Multivariate Behav Res
Bianconi, Violi, Fallarino, Pignatelli, Sahebkar et al., Is Acetylsalicylic acid a safe and potentially useful choice for adult patients with COVID-19 ?, Drugs
Chow, Khanna, Kethireddy, Aspirin use is associated with decreased mechanical ventilation, ICU admission, and in-hospital mortality in hospitalized patients with COVID-19, Anesth Analg
Coulombe, Jaworska, Verway, Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages, Immunity
Day, Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists, BMJ
Dotolo, Marabotti, Facchiano, Tagliaferri, A review on drug repurposing applicable to COVID-19, Brief Bioinform
Eisen, Leder, Woods, Effect of aspirin on deaths associated with sepsis in healthy older people (ANTISEPSIS): a randomised, doubleblind, placebo-controlled primary prevention trial, Lancet Respir Med
Evangelista, Manarini, Dell'elba, Clopidogrel inhibits platelet-leukocyte adhesion and platelet-dependent leukocyte activation, Thromb Haemost
Giannis, Ziogas, Gianni, Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past, J Clin Virol
Glatthaar-Saalmüller, Mair, Saalmüller, Antiviral activity of aspirin against RNA viruses of the respiratory tract-an in vitro study, Influenza Other Respir Viruses
Gupta, Madhavan, Sehgal, Extrapulmonary manifestations of COVID-19, Nat Med
Harbi, Tamim, Al-Dorzi, Sadat, Arabi, Association between aspirin therapy and the outcome in critically ill patients: a nested cohort study, BMC Pharmacol Toxicol
Hong, Lee, Kim, Guidelines for laboratory diagnosis of coronavirus disease 2019 (COVID-19) in Korea, Ann Lab Med
Hsu, Donnelly, Chaudhary, Aspirin use and long-term rates of sepsis:a population-based cohort study, PLoS One
Jeong, Lee, Shin, Choe, Filion et al., Association between NSAIDs use and adverse clinical outcomes among adults hospitalized with COVID-19 in South Korea: a nationwide study, Clin Infect Dis
Jung, Choi, You, Kim, Association of renin-angiotensinaldosterone system inhibitors with COVID-19-related outcomes in Korea: a nationwide population-based cohort study, Clin Infect Dis
Kwon, Thirty years of national health insurance in South Korea: lessons for achieving universal health care coverage, Health Policy Plan
Levey, Bosch, Lewis, Greene, Rogers et al., A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group, Ann Intern Med
Mehta, Mcauley, Brown, Sanchez, Tattersall et al., COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet
Morris, Stables, Hobbs, Effects of low-dose aspirin on acute inflammatory responses in humans, J Immunol
Pamukcu, Inflammation and thrombosis in patients with COVID-19: a prothrombotic and inflammatory disease caused by SARS coronavirus-2, Anatol J Cardiol
Park, Choi, Ko, Information technology-based tracing strategy in response to COVID-19 in South Korea-Privacy Controversies, JAMA
Quan, Sundararajan, Halfon, Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data, Med Care
Rajasagi, Bhela, Varanasi, Rouse, Frontline Science: Aspirin-triggered resolvin D1 controls herpes simplex virus-induced corneal immunopathology, J Leukoc Biol
Shim, Tariq, Choi, Lee, Chowell, Transmission potential and severity of COVID-19 in South Korea, Int J Infect Dis
Singh, Parida, Lingaraju, Kesavan, Kumar et al., Drug repurposing approach to fight COVID-19, Pharmacol Rep
Son, Seo, Yang, Association between renin-angiotensinaldosterone system inhibitors and COVID-19 infection in South Korea, Hypertension
Song, Jung, Song, Background and data configuration process of a nationwide population-based study using the korean national health insurance system, Diabetes Metab J
Sultana, Crisafulli, Gabbay, Lynn, Shakir et al., Challenges for drug repurposing in the COVID-19 pandemic era, Front Pharmacol
Terpos, Ntanasis-Stathopoulos, Elalamy, Hematological findings and complications of COVID-19, Am J Hematol
Voiriot, Chalumeau, Messika, Risks associated with the use of non-steroidal anti-inflammatory drugs during pneumonia, Rev Mal Respir
Warner, Nylander, Whatling, Anti-platelet therapy: cyclooxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy, Br J Clin Pharmacol
Wiersinga, Rhodes, Cheng, Peacock, Prescott, Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review, JAMA
Wiewel, De Stoppelaar, Van Vught, Chronic antiplatelet therapy is not associated with alterations in the presentation, outcome, or host response biomarkers during sepsis: a propensity-matched analysis, Intensive Care Med
Yin, Yamamoto, Gaynor, The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta, Nature
Yuan, Chen, Li, Chen, Wang et al., Mortality and prehospitalization use of low-dose aspirin in COVID-19 patients with coronary artery disease, J Cell Mol Med
Zhang, Kim, Lonjon, Zhu, written on behalf of AMEB-DCTCGBalance diagnostics after propensity score matching, Ann Transl Med
DOI record:
{
"DOI": "10.1097/md.0000000000026670",
"ISSN": [
"0025-7974",
"1536-5964"
],
"URL": "http://dx.doi.org/10.1097/md.0000000000026670",
"author": [
{
"affiliation": [],
"family": "Son",
"given": "Minkook",
"sequence": "first"
},
{
"affiliation": [],
"family": "Noh",
"given": "Myung-giun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Jeong Hoon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Seo",
"given": "Jeongkuk",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Park",
"given": "Hansoo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Sung",
"sequence": "additional"
}
],
"container-title": [
"Medicine"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
8,
16
]
],
"date-time": "2021-08-16T15:43:05Z",
"timestamp": 1629128585000
},
"deposited": {
"date-parts": [
[
2021,
8,
26
]
],
"date-time": "2021-08-26T00:06:56Z",
"timestamp": 1629936416000
},
"funder": [
{
"DOI": "10.13039/501100003725",
"award": [
"2020R1A5A8018367"
],
"doi-asserted-by": "crossref",
"name": "National Research Foundation of Korea"
},
{
"DOI": "10.13039/501100003725",
"award": [
"2020R1A5A8018367"
],
"doi-asserted-by": "crossref",
"name": "National Research Foundation of Korea"
},
{
"DOI": "10.13039/501100003725",
"award": [
"2021R1A2C3008169"
],
"doi-asserted-by": "crossref",
"name": "National Research Foundation of Korea"
},
{
"DOI": "10.13039/501100003725",
"award": [
"2021R1A2C3008169"
],
"doi-asserted-by": "crossref",
"name": "National Research Foundation of Korea"
}
],
"indexed": {
"date-parts": [
[
2022,
1,
7
]
],
"date-time": "2022-01-07T02:50:57Z",
"timestamp": 1641523857772
},
"is-referenced-by-count": 1,
"issn-type": [
{
"type": "print",
"value": "0025-7974"
},
{
"type": "electronic",
"value": "1536-5964"
}
],
"issue": "30",
"issued": {
"date-parts": [
[
2021,
7,
30
]
]
},
"journal-issue": {
"issue": "30",
"published-print": {
"date-parts": [
[
2021
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://journals.lww.com/10.1097/MD.0000000000026670",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "276",
"original-title": [],
"page": "e26670",
"prefix": "10.1097",
"published": {
"date-parts": [
[
2021,
7,
30
]
]
},
"published-online": {
"date-parts": [
[
2021,
7,
30
]
]
},
"publisher": "Ovid Technologies (Wolters Kluwer Health)",
"reference": [
{
"DOI": "10.1038/s41591-020-0968-3",
"article-title": "Extrapulmonary manifestations of COVID-19",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1017",
"journal-title": "Nat Med",
"key": "R2-20210825",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"article-title": "COVID-19: consider cytokine storm syndromes and immunosuppression",
"author": "Mehta",
"doi-asserted-by": "crossref",
"first-page": "1033",
"journal-title": "Lancet",
"key": "R3-20210825",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1002/ajh.25829",
"article-title": "Hematological findings and complications of COVID-19",
"author": "Terpos",
"doi-asserted-by": "crossref",
"first-page": "834",
"journal-title": "Am J Hematol",
"key": "R4-20210825",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.1016/j.jcv.2020.104362",
"article-title": "Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past",
"author": "Giannis",
"doi-asserted-by": "crossref",
"first-page": "104362",
"journal-title": "J Clin Virol",
"key": "R5-20210825",
"volume": "127",
"year": "2020"
},
{
"DOI": "10.1111/j.1365-2125.2011.03943.x",
"article-title": "Anti-platelet therapy: cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy",
"author": "Warner",
"doi-asserted-by": "crossref",
"first-page": "619",
"journal-title": "Br J Clin Pharmacol",
"key": "R6-20210825",
"volume": "72",
"year": "2011"
},
{
"DOI": "10.1007/s40265-020-01365-1",
"article-title": "Is Acetylsalicylic acid a safe and potentially useful choice for adult patients with COVID-19 ?",
"author": "Bianconi",
"doi-asserted-by": "crossref",
"first-page": "1383",
"journal-title": "Drugs",
"key": "R7-20210825",
"volume": "80",
"year": "2020"
},
{
"DOI": "10.1111/irv.12421",
"article-title": "Antiviral activity of aspirin against RNA viruses of the respiratory tract-an in vitro study",
"author": "Glatthaar-Saalmüller",
"doi-asserted-by": "crossref",
"first-page": "85",
"journal-title": "Influenza Other Respir Viruses",
"key": "R8-20210825",
"volume": "11",
"year": "2017"
},
{
"article-title": "Inflammation and thrombosis in patients with COVID-19: a prothrombotic and inflammatory disease caused by SARS coronavirus-2",
"author": "Pamukcu",
"first-page": "224",
"journal-title": "Anatol J Cardiol",
"key": "R9-20210825",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1016/j.mehy.2020.109975",
"article-title": "Should aspirin be used for prophylaxis of COVID-19-induced coagulopathy?",
"author": "Mohamed-Hussein",
"doi-asserted-by": "crossref",
"first-page": "109975",
"journal-title": "Med Hypotheses",
"key": "R10-20210825",
"volume": "144",
"year": "2020"
},
{
"DOI": "10.1213/ANE.0000000000005292",
"article-title": "Aspirin use is associated with decreased mechanical ventilation, ICU admission, and in-hospital mortality in hospitalized patients with COVID-19",
"author": "Chow",
"doi-asserted-by": "crossref",
"first-page": "930",
"journal-title": "Anesth Analg",
"key": "R11-20210825",
"volume": "132",
"year": "2021"
},
{
"DOI": "10.1111/jcmm.16198",
"article-title": "Mortality and pre-hospitalization use of low-dose aspirin in COVID-19 patients with coronary artery disease",
"author": "Yuan",
"doi-asserted-by": "crossref",
"first-page": "1263",
"journal-title": "J Cell Mol Med",
"key": "R12-20210825",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1093/heapol/czn037",
"article-title": "Thirty years of national health insurance in South Korea: lessons for achieving universal health care coverage",
"author": "Kwon",
"doi-asserted-by": "crossref",
"first-page": "63",
"journal-title": "Health Policy Plan",
"key": "R13-20210825",
"volume": "24",
"year": "2009"
},
{
"DOI": "10.4093/dmj.2014.38.5.395",
"article-title": "Background and data configuration process of a nationwide population-based study using the korean national health insurance system",
"author": "Song",
"doi-asserted-by": "crossref",
"first-page": "395",
"journal-title": "Diabetes Metab J",
"key": "R14-20210825",
"volume": "38",
"year": "2014"
},
{
"DOI": "10.1001/jama.2020.6602",
"article-title": "Information technology-based tracing strategy in response to COVID-19 in South Korea-Privacy Controversies",
"author": "Park",
"doi-asserted-by": "crossref",
"first-page": "2129",
"journal-title": "JAMA",
"key": "R15-20210825",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.3343/alm.2020.40.5.351",
"article-title": "Guidelines for laboratory diagnosis of coronavirus disease 2019 (COVID-19) in Korea",
"author": "Hong",
"doi-asserted-by": "crossref",
"first-page": "351",
"journal-title": "Ann Lab Med",
"key": "R16-20210825",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa624",
"article-title": "Association of renin-angiotensin-aldosterone system inhibitors with COVID-19-related outcomes in Korea: a nationwide population-based cohort study",
"author": "Jung",
"doi-asserted-by": "crossref",
"first-page": "2121",
"journal-title": "Clin Infect Dis",
"key": "R17-20210825",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1161/HYPERTENSIONAHA.120.15464",
"article-title": "Association between renin-angiotensin-aldosterone system inhibitors and COVID-19 infection in South Korea",
"author": "Son",
"doi-asserted-by": "crossref",
"first-page": "742",
"journal-title": "Hypertension",
"key": "R18-20210825",
"volume": "76",
"year": "2020"
},
{
"article-title": "Association between NSAIDs use and adverse clinical outcomes among adults hospitalized with COVID-19 in South Korea: a nationwide study",
"author": "Jeong",
"journal-title": "Clin Infect Dis",
"key": "R19-20210825",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.03.031",
"article-title": "Transmission potential and severity of COVID-19 in South Korea",
"author": "Shim",
"doi-asserted-by": "crossref",
"first-page": "339",
"journal-title": "Int J Infect Dis",
"key": "R20-20210825",
"volume": "93",
"year": "2020"
},
{
"DOI": "10.1097/01.mlr.0000182534.19832.83",
"article-title": "Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data",
"author": "Quan",
"doi-asserted-by": "crossref",
"first-page": "1130",
"journal-title": "Med Care",
"key": "R21-20210825",
"volume": "43",
"year": "2005"
},
{
"DOI": "10.7326/0003-4819-130-6-199903160-00002",
"article-title": "A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group",
"author": "Levey",
"doi-asserted-by": "crossref",
"first-page": "461",
"journal-title": "Ann Intern Med",
"key": "R22-20210825",
"volume": "130",
"year": "1999"
},
{
"DOI": "10.1080/00273171.2011.568786",
"article-title": "An Introduction to propensity score methods for reducing the effects of confounding in observational studies",
"author": "Austin",
"doi-asserted-by": "crossref",
"first-page": "399",
"journal-title": "Multivariate Behav Res",
"key": "R23-20210825",
"volume": "46",
"year": "2011"
},
{
"DOI": "10.21037/atm.2018.12.10",
"article-title": "Balance diagnostics after propensity score matching",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "16",
"journal-title": "Ann Transl Med",
"key": "R24-20210825",
"volume": "7",
"year": "2019"
},
{
"DOI": "10.1016/j.immuni.2014.02.013",
"article-title": "Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages",
"author": "Coulombe",
"doi-asserted-by": "crossref",
"first-page": "554",
"journal-title": "Immunity",
"key": "R25-20210825",
"volume": "40",
"year": "2014"
},
{
"DOI": "10.1189/jlb.3HI1216-511RR",
"article-title": "Frontline Science: Aspirin-triggered resolvin D1 controls herpes simplex virus-induced corneal immunopathology",
"author": "Rajasagi",
"doi-asserted-by": "crossref",
"first-page": "1159",
"journal-title": "J Leukoc Biol",
"key": "R26-20210825",
"volume": "102",
"year": "2017"
},
{
"DOI": "10.1001/jama.2020.12839",
"article-title": "Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review",
"author": "Wiersinga",
"doi-asserted-by": "crossref",
"first-page": "782",
"journal-title": "JAMA",
"key": "R27-20210825",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1007/s00134-020-06022-5",
"article-title": "Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19)",
"author": "Alhazzani",
"doi-asserted-by": "crossref",
"first-page": "854",
"journal-title": "Intensive Care Med",
"key": "R28-20210825",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1186/s40360-016-0047-z",
"article-title": "Association between aspirin therapy and the outcome in critically ill patients: a nested cohort study",
"author": "Al Harbi",
"doi-asserted-by": "crossref",
"first-page": "05",
"journal-title": "BMC Pharmacol Toxicol",
"key": "R29-20210825",
"volume": "17",
"year": "2016"
},
{
"DOI": "10.1007/s00134-015-4171-9",
"article-title": "Chronic antiplatelet therapy is not associated with alterations in the presentation, outcome, or host response biomarkers during sepsis: a propensity-matched analysis",
"author": "Wiewel",
"doi-asserted-by": "crossref",
"first-page": "352",
"journal-title": "Intensive Care Med",
"key": "R30-20210825",
"volume": "42",
"year": "2016"
},
{
"DOI": "10.1371/journal.pone.0194829",
"article-title": "Aspirin use and long-term rates of sepsis:a population-based cohort study",
"author": "Hsu",
"doi-asserted-by": "crossref",
"first-page": "e0194829",
"journal-title": "PLoS One",
"key": "R31-20210825",
"volume": "13",
"year": "2018"
},
{
"DOI": "10.1016/S2213-2600(20)30411-2",
"article-title": "Effect of aspirin on deaths associated with sepsis in healthy older people (ANTISEPSIS): a randomised, double-blind, placebo-controlled primary prevention trial",
"author": "Eisen",
"doi-asserted-by": "crossref",
"first-page": "186",
"journal-title": "Lancet Respir Med",
"key": "R32-20210825",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1160/TH05-01-0020",
"article-title": "Clopidogrel inhibits platelet-leukocyte adhesion and platelet-dependent leukocyte activation",
"author": "Evangelista",
"doi-asserted-by": "crossref",
"first-page": "568",
"journal-title": "Thromb Haemost",
"key": "R33-20210825",
"volume": "94",
"year": "2005"
},
{
"DOI": "10.4049/jimmunol.0900477",
"article-title": "Effects of low-dose aspirin on acute inflammatory responses in humans",
"author": "Morris",
"doi-asserted-by": "crossref",
"first-page": "2089",
"journal-title": "J Immunol",
"key": "R34-20210825",
"volume": "183",
"year": "2009"
},
{
"DOI": "10.1038/23948",
"article-title": "The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta",
"author": "Yin",
"doi-asserted-by": "crossref",
"first-page": "77",
"journal-title": "Nature",
"key": "R35-20210825",
"volume": "396",
"year": "1998"
},
{
"DOI": "10.1136/bmj.m1086",
"article-title": "Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists",
"author": "Day",
"doi-asserted-by": "crossref",
"first-page": "m1086",
"journal-title": "BMJ",
"key": "R36-20210825",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1016/j.rmr.2017.12.003",
"article-title": "[Risks associated with the use of non-steroidal anti-inflammatory drugs during pneumonia]",
"author": "Voiriot",
"doi-asserted-by": "crossref",
"first-page": "430",
"journal-title": "Rev Mal Respir",
"key": "R37-20210825",
"volume": "35",
"year": "2018"
},
{
"DOI": "10.1007/s43440-020-00155-6",
"article-title": "Drug repurposing approach to fight COVID-19",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "1479",
"journal-title": "Pharmacol Rep",
"key": "R38-20210825",
"volume": "72",
"year": "2020"
},
{
"DOI": "10.3389/fphar.2020.588654",
"article-title": "Challenges for drug repurposing in the COVID-19 pandemic era",
"author": "Sultana",
"doi-asserted-by": "crossref",
"first-page": "588654",
"journal-title": "Front Pharmacol",
"key": "R39-20210825",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1093/bib/bbaa288",
"article-title": "A review on drug repurposing applicable to COVID-19",
"author": "Dotolo",
"doi-asserted-by": "crossref",
"first-page": "726",
"journal-title": "Brief Bioinform",
"key": "R40-20210825",
"volume": "22",
"year": "2021"
}
],
"reference-count": 39,
"references-count": 39,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [
"A nationwide case-control study in South Korea"
],
"title": [
"Effect of aspirin on coronavirus disease 2019"
],
"type": "journal-article",
"volume": "100"
}