Use of non-steroidal anti-inflammatory drugs and risk of death from COVID-19: an OpenSAFELY cohort analysis based on two cohorts
et al., Annals of the Rheumatic Diseases, doi:10.1136/annrheumdis-2020-219517, Jan 2021
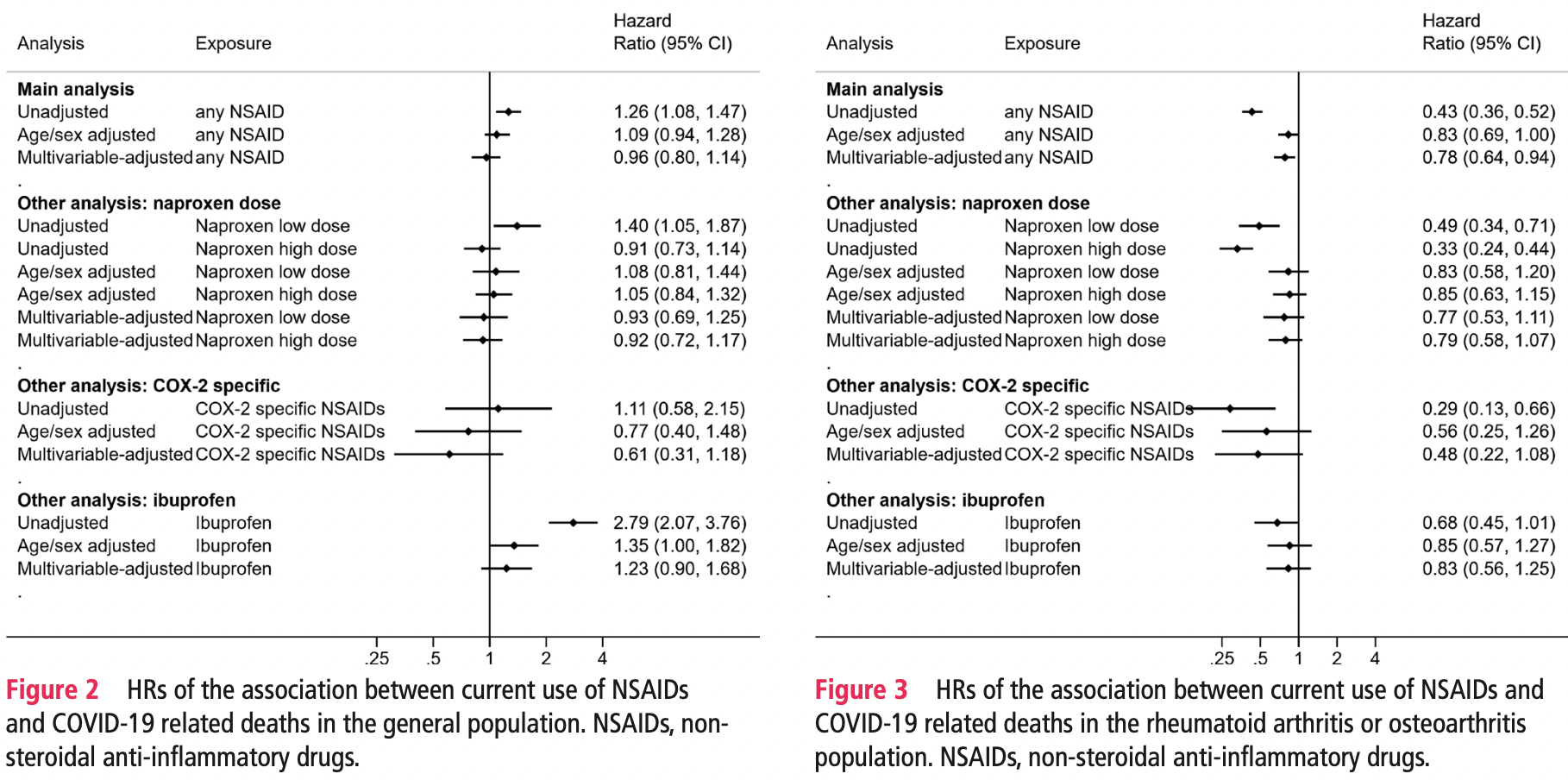
Retrospective 2,463,707 people in the UK, showing no significant difference in COVID-19 mortality with NSAID use. Current NSAID users were defined as those ever prescribed an NSAID in the 4 months prior to study start, and non-users were those with no record of NSAID prescription in the same time period.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments1.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 23.0% higher, HR 1.23, p = 0.19, adjusted per study, general population, multivariable.
|
|
risk of death, 17.0% lower, HR 0.83, p = 0.37, adjusted per study, rheumatoid arthritis/osteoarthritis patients, multivariable.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Wong et al., 21 Jan 2021, retrospective, United Kingdom, peer-reviewed, median age 53.0, 32 authors, study period 1 March, 2020 - 14 June, 2020.
Use of non-steroidal anti-inflammatory drugs and risk of death from COVID-19: an OpenSAFELY cohort analysis based on two cohorts
Annals of the Rheumatic Diseases, doi:10.1136/annrheumdis-2020-219517
Objectives To assess the association between routinely prescribed non-steroidal anti-inflammatory drugs (NSAIDs) and deaths from COVID-19 using OpenSAFELY, a secure analytical platform. Methods We conducted two cohort studies from 1 March to 14 June 2020. Working on behalf of National Health Service England, we used routine clinical data in England linked to death data. In study 1, we identified people with an NSAID prescription in the last 3 years from the general population. In study 2, we identified people with rheumatoid arthritis/osteoarthritis. We defined exposure as current NSAID prescription within the 4 months before 1 March 2020. We used Cox regression to estimate HRs for COVID-19 related death in people currently prescribed NSAIDs, compared with those not currently prescribed NSAIDs, accounting for age, sex, comorbidities, other medications and geographical region.
Results In study 1, we included 536 423 current NSAID users and 1 927 284 non-users in the general population. We observed no evidence of difference in risk of COVID-19 related death associated with current use (HR 0.96, 95% CI 0.80 to 1.14) in the multivariable-adjusted model. In study 2, we included 1 708 781 people with rheumatoid arthritis/osteoarthritis, of whom 175 495 (10%) were current NSAID users. In the multivariable-adjusted model, we observed a lower risk of COVID-19 related death (HR 0.78, 95% CI 0.64 to 0.94) associated with current use of NSAID versus non-use. Conclusions We found no evidence of a harmful effect of routinely prescribed NSAIDs on COVID-19 related deaths. Risks of COVID-19 do not need to influence decisions about the routine therapeutic use of NSAIDs.
INTRODUCTION COVID-19, caused by the SARS-CoV-2, has been diagnosed in approximately 18 million patients with >690 000 deaths in >200 countries as of 5 August 2020. 1
Key messages
What is already known about this subject? ► There have been concerns that non-steroidal anti-inflammatory drugs (NSAIDs) may increase the risk of COVID-19 disease. Recent observational studies reported no evidence of a harmful effect of NSAID use on COVID-19 severity among patients with COVID-19. ► However, most studies were of much smaller sample size, not general population based or did not specifically investigate individual NSAIDs (eg, naproxen and ibuprofen). ► In addition, limited clinical data are available to advise patients using long-term NSAID treatment (including people with rheumatoid arthritis and osteoarthritis) whether the treatment should be continued or stopped in the context of COVID-19 pandemic.
What does this study add? ► We identified two study populations (2 463 707 people who ever used NSAIDs in the past 3 years from the general population and 1 708 781 people with rheumatoid arthritis/ osteoarthritis) in England using OpenSAFELY platform. We then grouped them into current users and non-users, respectively, in each study population. ► In both populations, no association between..
Abbreviations: COPD, Chronic obstructive pulmonary disease; DMARDs, Disease-modifying antirheumatic drugs. BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s)
References
Amici, Caro, Ciucci, Indomethacin has a potent antiviral activity against SARS coronavirus, Antivir Ther
Bancos, Bernard, Topham, Ibuprofen and other widely used nonsteroidal anti-inflammatory drugs inhibit antibody production in human cells, Cell Immunol, doi:10.1016/j.cellimm.2009.03.007
Basille, Thomsen, Madsen, Nonsteroidal antiinflammatory drug use and clinical outcomes of community-acquired pneumonia, Am J Respir Crit Care Med, doi:10.1164/rccm.201802-0229LE
Bourgeois, Ferroni, Leruez-Ville, Nonsteroidal anti-inflammatory drug without antibiotics for acute viral infection increases the empyema risk in children: a matched case-control study, J Pediatr, doi:10.1016/j.jpeds.2016.05.025
Bruce, Barlow-Pay, Short, Prior routine use of non-steroidal antiinflammatory drugs (NSAIDs) and important outcomes in hospitalised patients with COVID-19, J Clin Med, doi:10.3390/jcm9082586
Byington, Spencer, Johnson, An epidemiological investigation of a sustained high rate of pediatric parapneumonic empyema: risk factors and microbiological associations, Clin Infect Dis, doi:10.1086/338460
Choi, Ahn, Ryu, Clinical characteristics and disease progression in early-stage COVID-19 patients in South Korea, J Clin Med, doi:10.3390/jcm9061959
Clinicaltrials, Gov, Efficacy of addition of naproxen in the treatment of critically ill patients hospitalized for COVID-19 infection
Clinicaltrials, Gov, Evaluate the efficacy and safety of oral hydroxychloroquine, indomethacin and Zithromax in subjects with mild symptoms of COVID-19
Clinicaltrials, Gov, Inhaled ibuprofen to treat COVID-19
Clinicaltrials, Liberate trial in COVID-19
Ding, Vanderweele, Sensitivity analysis without assumptions, Epidemiology, doi:10.1097/EDE.0000000000000457
Fang, Karakiulakis, Roth, Gu, Xie et al., Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?, Lancet Respir Med, doi:10.1038/srep19840
Francisco, EMA gives advice on the use of non-steroidal anti-inflammatories for COVID-19 -European Medicines Agency
François, Desrumaux, Cans, Prevalence and risk factors of suppurative complications in children with pneumonia, Acta Paediatr, doi:10.1111/j.1651-2227.2010.01734.x
Gianfrancesco, Hyrich, Al-Adely, Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry, Ann Rheum Dis, doi:10.1136/annrheumdis-2020-217871
Goldacre, Mackenna, The NHS deserves better use of hospital medicines data, BMJ, doi:10.1136/bmj.m2607
Imam, Odish, Gill, Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States, J Intern Med, doi:10.1111/joim.13119
Kaplan, Edelson, Korchak, Effects of non-steroidal anti-inflammatory agents on human neutrophil functions in vitro and in vivo, Biochem Pharmacol, doi:10.1016/0006-2952(84)90228-4
Kotsiou, Zarogiannis, Gourgoulianis, Prehospital NSAIDs use prolong hospitalization in patients with pleuro-pulmonary infection, Respir Med, doi:10.1016/j.rmed.2016.12.005
Little, Moore, Kelly, Ibuprofen, paracetamol, and steam for patients with respiratory tract infections in primary care: pragmatic randomised factorial trial, BMJ, doi:10.1136/bmj.f6041
Lund, Kristensen, Reilev, Adverse outcomes and mortality in users of non-steroidal anti-inflammatory drugs who tested positive for SARS-CoV-2: a Danish nationwide cohort study, PLoS Med, doi:10.1371/journal.pmed.1003308
Mancia, Rea, Ludergnani, Renin-Angiotensin-Aldosterone system blockers and the risk of Covid-19, N Engl J Med, doi:10.1056/NEJMoa2006923
Matthews, Donaldson, Evans, Safety of medicines delivered by homecare companies, BMJ, doi:10.1136/bmj.k2201
Messika, Sztrymf, Bertrand, Risks of nonsteroidal antiinflammatory drugs in undiagnosed intensive care unit pneumococcal pneumonia: younger and more severely affected patients, J Crit Care, doi:10.1016/j.jcrc.2014.05.021
Nhs England, Guidance on conditions for which over the counter items should not routinely be prescribed in primary care
Nhsengland, Local sustainability and transformation partnership
Qiao, Wang, Chen, Ibuprofen attenuates cardiac fibrosis in streptozotocininduced diabetic rats, Cardiology, doi:10.1159/000375362
Rheum Dis, 58mmols/mol) Uncontrolled (HbA1c ≥ 58mmols, /mol)
Rheum Dis, 58mmols/mol) Uncontrolled (HbA1c ≥ 58mmols, /mol)
Rheum Dis, 58mmols/mol) Uncontrolled (HbA1c ≥ 58mmols/mol)
Rheum Dis, 58mmols/mol) Uncontrolled (HbA1c ≥ 58mmols/mol)
Rheum Dis, Controlled (HbA1c < 58mmols, /mol)
Rinott, Kozer, Shapira, Ibuprofen use and clinical outcomes in COVID-19 patients, Clin Microbiol Infect, doi:10.1016/j.cmi.2020.06.003
Vaja, Chan, Ferreira, The COVID-19 ibuprofen controversy: a systematic review of NSAIDs in adult acute lower respiratory tract infections, Br J Clin Pharmacol, doi:10.1111/bcp.14514
Voiriot, Dury, Parrot, Nonsteroidal antiinflammatory drugs may affect the presentation and course of community-acquired pneumonia, Chest, doi:10.1378/chest.09-3102
Voiriot, Philippot, Elabbadi, Risks related to the use of non-steroidal anti-inflammatory drugs in community-acquired pneumonia in adult and pediatric patients, J Clin Med, doi:10.3390/jcm8060786
Who, Emergency use ICD codes for COVID-19 disease outbreak
Williamson, Walker, Bhaskaran, Factors associated with COVID-19-related death using OpenSAFELY, Nature, doi:10.1038/s41586-020-2521-4
Yousefifard, Zali, Zarghi, Non-Steroidal anti-inflammatory drugs in management of COVID-19; a systematic review on current evidence, Int J Clin Pract, doi:10.1111/ijcp.13557
Zou, Yan, Shu, Angiotensin-Converting enzyme 2 protects from lethal avian influenza A H5N1 infections, Nat Commun, doi:10.1038/ncomms4594
DOI record:
{
"DOI": "10.1136/annrheumdis-2020-219517",
"ISSN": [
"0003-4967",
"1468-2060"
],
"URL": "http://dx.doi.org/10.1136/annrheumdis-2020-219517",
"abstract": "<jats:sec><jats:title>Objectives</jats:title><jats:p>To assess the association between routinely prescribed non-steroidal anti-inflammatory drugs (NSAIDs) and deaths from COVID-19 using OpenSAFELY, a secure analytical platform.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>We conducted two cohort studies from 1 March to 14 June 2020. Working on behalf of National Health Service England, we used routine clinical data in England linked to death data. In study 1, we identified people with an NSAID prescription in the last 3 years from the general population. In study 2, we identified people with rheumatoid arthritis/osteoarthritis. We defined exposure as current NSAID prescription within the 4 months before 1 March 2020. We used Cox regression to estimate HRs for COVID-19 related death in people currently prescribed NSAIDs, compared with those not currently prescribed NSAIDs, accounting for age, sex, comorbidities, other medications and geographical region.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>In study 1, we included 536 423 current NSAID users and 1 927 284 non-users in the general population. We observed no evidence of difference in risk of COVID-19 related death associated with current use (HR 0.96, 95% CI 0.80 to 1.14) in the multivariable-adjusted model. In study 2, we included 1 708 781 people with rheumatoid arthritis/osteoarthritis, of whom 175 495 (10%) were current NSAID users. In the multivariable-adjusted model, we observed a lower risk of COVID-19 related death (HR 0.78, 95% CI 0.64 to 0.94) associated with current use of NSAID versus non-use.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>We found no evidence of a harmful effect of routinely prescribed NSAIDs on COVID-19 related deaths. Risks of COVID-19 do not need to influence decisions about the routine therapeutic use of NSAIDs.</jats:p></jats:sec>",
"alternative-id": [
"10.1136/annrheumdis-2020-219517"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-8618-7333",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wong",
"given": "Angel YS",
"sequence": "first"
},
{
"affiliation": [],
"family": "MacKenna",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morton",
"given": "Caroline E",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schultze",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Walker",
"given": "Alex J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bhaskaran",
"given": "Krishnan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brown",
"given": "Jeremy P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rentsch",
"given": "Christopher T",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Williamson",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Drysdale",
"given": "Henry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Croker",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bacon",
"given": "Seb",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hulme",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bates",
"given": "Chris",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Curtis",
"given": "Helen J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mehrkar",
"given": "Amir",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Evans",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Inglesby",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cockburn",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McDonald",
"given": "Helen I",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tomlinson",
"given": "Laurie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mathur",
"given": "Rohini",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wing",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Forbes",
"given": "Harriet",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Eggo",
"given": "Rosalind M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parry",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hester",
"given": "Frank",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harper",
"given": "Sam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Evans",
"given": "Stephen JW",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smeeth",
"given": "Liam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Douglas",
"given": "Ian J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goldacre",
"given": "Ben",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct04325633",
"registry": "10.18810/clinical-trials-gov"
},
{
"clinical-trial-number": "nct04382768",
"registry": "10.18810/clinical-trials-gov"
},
{
"clinical-trial-number": "nct04334629",
"registry": "10.18810/clinical-trials-gov"
},
{
"clinical-trial-number": "nct04344457",
"registry": "10.18810/clinical-trials-gov"
},
{
"clinical-trial-number": "nct04325633",
"registry": "10.18810/clinical-trials-gov"
},
{
"clinical-trial-number": "nct04382768",
"registry": "10.18810/clinical-trials-gov"
},
{
"clinical-trial-number": "nct04334629",
"registry": "10.18810/clinical-trials-gov"
},
{
"clinical-trial-number": "nct04344457",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": "Annals of the Rheumatic Diseases",
"container-title-short": "Ann Rheum Dis",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bmj.com"
]
},
"created": {
"date-parts": [
[
2021,
1,
21
]
],
"date-time": "2021-01-21T18:29:53Z",
"timestamp": 1611253793000
},
"deposited": {
"date-parts": [
[
2022,
7,
4
]
],
"date-time": "2022-07-04T16:24:21Z",
"timestamp": 1656951861000
},
"funder": [
{
"DOI": "10.13039/501100000265",
"award": [
"MR/V015737/1"
],
"doi-asserted-by": "publisher",
"name": "Medical Research Council"
}
],
"indexed": {
"date-parts": [
[
2022,
8,
8
]
],
"date-time": "2022-08-08T16:28:13Z",
"timestamp": 1659976093957
},
"is-referenced-by-count": 30,
"issue": "7",
"issued": {
"date-parts": [
[
2021,
1,
21
]
]
},
"journal-issue": {
"issue": "7",
"published-online": {
"date-parts": [
[
2021,
6,
17
]
]
},
"published-print": {
"date-parts": [
[
2021,
7
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
1,
21
]
],
"date-time": "2021-01-21T00:00:00Z",
"timestamp": 1611187200000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1136/annrheumdis-2020-219517",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "239",
"original-title": [],
"page": "943-951",
"prefix": "10.1136",
"published": {
"date-parts": [
[
2021,
1,
21
]
]
},
"published-online": {
"date-parts": [
[
2021,
1,
21
]
]
},
"published-print": {
"date-parts": [
[
2021,
7
]
]
},
"publisher": "BMJ",
"reference": [
{
"key": "2022070409200593000_80.7.943.1",
"unstructured": "World Health Organization . Coronavirus disease (COVID-19) pandemic. Available: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [Accessed 2 Sep 2020]."
},
{
"key": "2022070409200593000_80.7.943.2",
"unstructured": "The DataLab . BNF 10.1.1: non-steroidal anti-inflammatory drugs | primary care prescriptions. OpenPrescribing. Available: https://openprescribing.net/bnf/100101/ [Accessed 13 Jul 2020]."
},
{
"key": "2022070409200593000_80.7.943.3",
"unstructured": "Pharmaceutical Journal . Breakdown of the OTC medicines market in Britain. Available: https://www.pharmaceutical-journal.com/news-and-analysis/infographics/breakdown-of-the-otc-medicines-market-in-britain/20204913.article [Accessed 13 Jul 2020]."
},
{
"DOI": "10.1007/s00408-016-9973-1",
"article-title": "Non-Steroidal anti-inflammatory drugs may worsen the course of community-acquired pneumonia: a cohort study",
"author": "Basille",
"doi-asserted-by": "crossref",
"first-page": "201",
"journal-title": "Lung",
"key": "2022070409200593000_80.7.943.4",
"volume": "195",
"year": "2017"
},
{
"DOI": "10.1164/rccm.201802-0229LE",
"doi-asserted-by": "publisher",
"key": "2022070409200593000_80.7.943.5"
},
{
"DOI": "10.1086/338460",
"doi-asserted-by": "publisher",
"key": "2022070409200593000_80.7.943.6"
},
{
"DOI": "10.1111/j.1651-2227.2010.01734.x",
"doi-asserted-by": "publisher",
"key": "2022070409200593000_80.7.943.7"
},
{
"DOI": "10.1016/j.rmed.2016.12.005",
"article-title": "Prehospital NSAIDs use prolong hospitalization in patients with pleuro-pulmonary infection",
"author": "Kotsiou",
"doi-asserted-by": "crossref",
"first-page": "28",
"journal-title": "Respir Med",
"key": "2022070409200593000_80.7.943.8",
"volume": "123",
"year": "2017"
},
{
"DOI": "10.1016/j.jpeds.2016.05.025",
"doi-asserted-by": "publisher",
"key": "2022070409200593000_80.7.943.9"
},
{
"DOI": "10.1016/j.jcrc.2014.05.021",
"doi-asserted-by": "publisher",
"key": "2022070409200593000_80.7.943.10"
},
{
"DOI": "10.1378/chest.09-3102",
"doi-asserted-by": "publisher",
"key": "2022070409200593000_80.7.943.11"
},
{
"DOI": "10.1136/bmj.f6041",
"doi-asserted-by": "publisher",
"key": "2022070409200593000_80.7.943.12"
},
{
"DOI": "10.1177/135965350601100803",
"article-title": "Indomethacin has a potent antiviral activity against SARS coronavirus",
"author": "Amici",
"doi-asserted-by": "crossref",
"first-page": "1021",
"journal-title": "Antivir Ther",
"key": "2022070409200593000_80.7.943.13",
"volume": "11",
"year": "2006"
},
{
"key": "2022070409200593000_80.7.943.14",
"unstructured": "Central Alerting System . Novel Coronavirus - Anti-inflammatory medications, 2020. Available: https://www.cas.mhra.gov.uk/ViewandAcknowledgment/ViewAlert.aspx?AlertID=103001 [Accessed 13 Jul 2020]."
},
{
"key": "2022070409200593000_80.7.943.15",
"unstructured": "Center for Drug Evaluation, Research . Fda advises patients on use of NSAIDs for COVID-19. U.S. food and drug administration, 2020. Available: https://www.fda.gov/drugs/drug-safety-and-availability/fda-advises-patients-use-non-steroidal-anti-inflammatory-drugs-nsaids-covid-19 [Accessed 27 Jul 2020]."
},
{
"key": "2022070409200593000_80.7.943.16",
"unstructured": "Medicines, Healthcare products Regulatory Agency . Commission on human medicines advice on ibuprofen and coronavirus (COVID-19), 2020. Available: https://www.gov.uk/government/news/commission-on-human-medicines-advice-on-ibuprofen-and-coronavirus-covid-19 [Accessed 27 Jul 2020]."
},
{
"key": "2022070409200593000_80.7.943.17",
"unstructured": "Francisco EM . EMA gives advice on the use of non-steroidal anti-inflammatories for COVID-19 - European Medicines Agency. European Medicines Agency, 2020. Available: https://www.ema.europa.eu/en/news/ema-gives-advice-use-non-steroidal-anti-inflammatories-covid-19 [Accessed 27 Jul 2020]."
},
{
"article-title": "The COVID-19 ibuprofen controversy: a systematic review of NSAIDs in adult acute lower respiratory tract infections",
"author": "Vaja",
"journal-title": "Br J Clin Pharmacol",
"key": "2022070409200593000_80.7.943.18",
"year": "2020"
},
{
"DOI": "10.1111/ijcp.13557",
"article-title": "Non-Steroidal anti-inflammatory drugs in management of COVID-19; a systematic review on current evidence",
"author": "Yousefifard",
"doi-asserted-by": "crossref",
"journal-title": "Int J Clin Pract",
"key": "2022070409200593000_80.7.943.19",
"volume": "74",
"year": "2020"
},
{
"DOI": "10.1371/journal.pmed.1003308",
"doi-asserted-by": "publisher",
"key": "2022070409200593000_80.7.943.20"
},
{
"DOI": "10.1038/s41586-020-2521-4",
"doi-asserted-by": "publisher",
"key": "2022070409200593000_80.7.943.21"
},
{
"key": "2022070409200593000_80.7.943.22",
"unstructured": "NICE-The National Institute for Health, Excellence C. BNF: British National Formulary - NICE. Available: https://bnf.nice.org.uk/drug/aspirin.html [Accessed 8 Oct 2020]."
},
{
"key": "2022070409200593000_80.7.943.23",
"unstructured": "WHO . Emergency use ICD codes for COVID-19 disease outbreak, 2020. Available: https://www.who.int/classifications/icd/covid19/en/ [Accessed 27 Jul 2020]."
},
{
"key": "2022070409200593000_80.7.943.24",
"unstructured": "NHSEngland . Local sustainability and transformation partnership. Available: https://www.england.nhs.uk/integratedcare/stps/view-stps/ [Accessed 11 Aug 2020]."
},
{
"DOI": "10.1097/EDE.0000000000000457",
"doi-asserted-by": "publisher",
"key": "2022070409200593000_80.7.943.25"
},
{
"DOI": "10.3390/jcm8060786",
"article-title": "Risks related to the use of non-steroidal anti-inflammatory drugs in community-acquired pneumonia in adult and pediatric patients",
"author": "Voiriot",
"doi-asserted-by": "crossref",
"journal-title": "J Clin Med",
"key": "2022070409200593000_80.7.943.26",
"volume": "8",
"year": "2019"
},
{
"DOI": "10.1016/0006-2952(84)90228-4",
"doi-asserted-by": "publisher",
"key": "2022070409200593000_80.7.943.27"
},
{
"DOI": "10.1016/j.cellimm.2009.03.007",
"doi-asserted-by": "publisher",
"key": "2022070409200593000_80.7.943.28"
},
{
"DOI": "10.1159/000375362",
"doi-asserted-by": "publisher",
"key": "2022070409200593000_80.7.943.29"
},
{
"DOI": "10.1016/S2213-2600(20)30116-8",
"article-title": "Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?",
"author": "Fang",
"doi-asserted-by": "crossref",
"journal-title": "Lancet Respir Med",
"key": "2022070409200593000_80.7.943.30",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1038/srep19840",
"doi-asserted-by": "publisher",
"key": "2022070409200593000_80.7.943.31"
},
{
"DOI": "10.1038/ncomms4594",
"doi-asserted-by": "publisher",
"key": "2022070409200593000_80.7.943.32"
},
{
"DOI": "10.3390/jcm9061959",
"article-title": "Clinical characteristics and disease progression in early-stage COVID-19 patients in South Korea",
"author": "Choi",
"doi-asserted-by": "crossref",
"journal-title": "J Clin Med",
"key": "2022070409200593000_80.7.943.33",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1136/annrheumdis-2020-217871",
"doi-asserted-by": "publisher",
"key": "2022070409200593000_80.7.943.34"
},
{
"DOI": "10.1016/j.cmi.2020.06.003",
"article-title": "Ibuprofen use and clinical outcomes in COVID-19 patients",
"author": "Rinott",
"doi-asserted-by": "crossref",
"first-page": "1259.e5",
"journal-title": "Clin Microbiol Infect",
"key": "2022070409200593000_80.7.943.35",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.3390/jcm9082586",
"article-title": "Prior routine use of non-steroidal anti-inflammatory drugs (NSAIDs) and important outcomes in hospitalised patients with COVID-19",
"author": "Bruce",
"doi-asserted-by": "crossref",
"first-page": "2586",
"journal-title": "J Clin Med",
"key": "2022070409200593000_80.7.943.36",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2006923",
"doi-asserted-by": "publisher",
"key": "2022070409200593000_80.7.943.37"
},
{
"DOI": "10.1111/joim.13119",
"article-title": "Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States",
"author": "Imam",
"doi-asserted-by": "crossref",
"first-page": "469",
"journal-title": "J Intern Med",
"key": "2022070409200593000_80.7.943.38",
"volume": "288",
"year": "2020"
},
{
"key": "2022070409200593000_80.7.943.39",
"unstructured": "ClinicalTrials.gov . Efficacy of addition of naproxen in the treatment of critically ill patients hospitalized for COVID-19 infection. Available: https://clinicaltrials.gov/ct2/show/NCT04325633?cond=NCT04325633&draw=2&rank=1 [Accessed 24 Jul 2020]."
},
{
"key": "2022070409200593000_80.7.943.40",
"unstructured": "ClinicalTrials.gov . Inhaled ibuprofen to treat COVID-19. Available: https://clinicaltrials.gov/ct2/show/NCT04382768?cond=NCT04382768&draw=2&rank=1"
},
{
"key": "2022070409200593000_80.7.943.41",
"unstructured": "ClinicalTrials.gov . Liberate trial in COVID-19. Available: https://clinicaltrials.gov/ct2/show/NCT04334629?cond=NCT04334629&draw=1&rank=1 [Accessed 24 Jul 2020]."
},
{
"key": "2022070409200593000_80.7.943.42",
"unstructured": "ClinicalTrials.gov . Evaluate the efficacy and safety of oral hydroxychloroquine, indomethacin and Zithromax in subjects with mild symptoms of COVID-19. Available: https://clinicaltrials.gov/ct2/show/NCT04344457?cond=NCT04344457&draw=1&rank=1 [Accessed 24 Jul 2020]."
},
{
"key": "2022070409200593000_80.7.943.43",
"unstructured": "NHS England . Guidance on conditions for which over the counter items should not routinely be prescribed in primary care. Available: https://www.england.nhs.uk/medicines-2/conditions-for-which-over-the-counter-items-should-not-routinely-be-prescribed/ [Accessed 13 Jul 2020]."
},
{
"key": "2022070409200593000_80.7.943.44",
"unstructured": "NHS England . NHS England drugs list - medicines not reimbursed through national prices. Available: https://www.england.nhs.uk/publication/nhs-england-drugs-list/ [Accessed 27 Jul 2020]."
},
{
"DOI": "10.1136/bmj.k2201",
"doi-asserted-by": "publisher",
"key": "2022070409200593000_80.7.943.45"
},
{
"DOI": "10.1136/bmj.m2607",
"doi-asserted-by": "publisher",
"key": "2022070409200593000_80.7.943.46"
},
{
"key": "2022070409200593000_80.7.943.47",
"unstructured": "NHS Digital . Beta – data security standards. Available: https://digital.nhs.uk/about-nhs-digital/our-work/nhs-digital-data-and-technology-standards/framework/beta---data-security-standards [Accessed 30 Apr 2020]."
},
{
"key": "2022070409200593000_80.7.943.48",
"unstructured": "NHS Digital . Data security and protection toolkit. Available: https://digital.nhs.uk/data-and-information/looking-after-information/data-security-and-information-governance/data-security-and-protection-toolkit [Accessed 30 Apr 2020]."
},
{
"key": "2022070409200593000_80.7.943.49",
"unstructured": "ISB1523: Anonymisation Standard for Publishing Health and Social Care Data - NHS Digital. Available: https://digital.nhs.uk/data-and-information/information-standards/information-standards-and-data-collections-including-extractions/publications-and-notifications/standards-and-collections/isb1523-anonymisation-standard-for-publishing-health-and-social-care-data [Accessed 30 Apr 2020]."
},
{
"key": "2022070409200593000_80.7.943.50",
"unstructured": "Secretary of State for Health and Social Care - UK Government . Coronavirus (COVID-19): notification to organisations to share information, 2020. Available: https://web.archive.org/web/20200421171727/https://www.gov.uk/government/publications/coronavirus-covid-19-notification-of-data-controllers-to-share-information"
}
],
"reference-count": 50,
"references-count": 50,
"relation": {},
"resource": {
"primary": {
"URL": "https://ard.bmj.com/lookup/doi/10.1136/annrheumdis-2020-219517"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Biochemistry, Genetics and Molecular Biology",
"Immunology",
"Immunology and Allergy",
"Rheumatology"
],
"subtitle": [],
"title": "Use of non-steroidal anti-inflammatory drugs and risk of death from COVID-19: an OpenSAFELY cohort analysis based on two cohorts",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1136/crossmarkpolicy",
"volume": "80"
}
wong4