A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19
et al., NEJM, June 3 2020, doi:10.1056/NEJMoa2016638, Jun 2020
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Remote post-exposure prophylaxis RCT reporting that "[HCQ] did not prevent illness compatible with Covid-19 or confirmed infection when used as postexposure prophylaxis within 4 days after exposure".
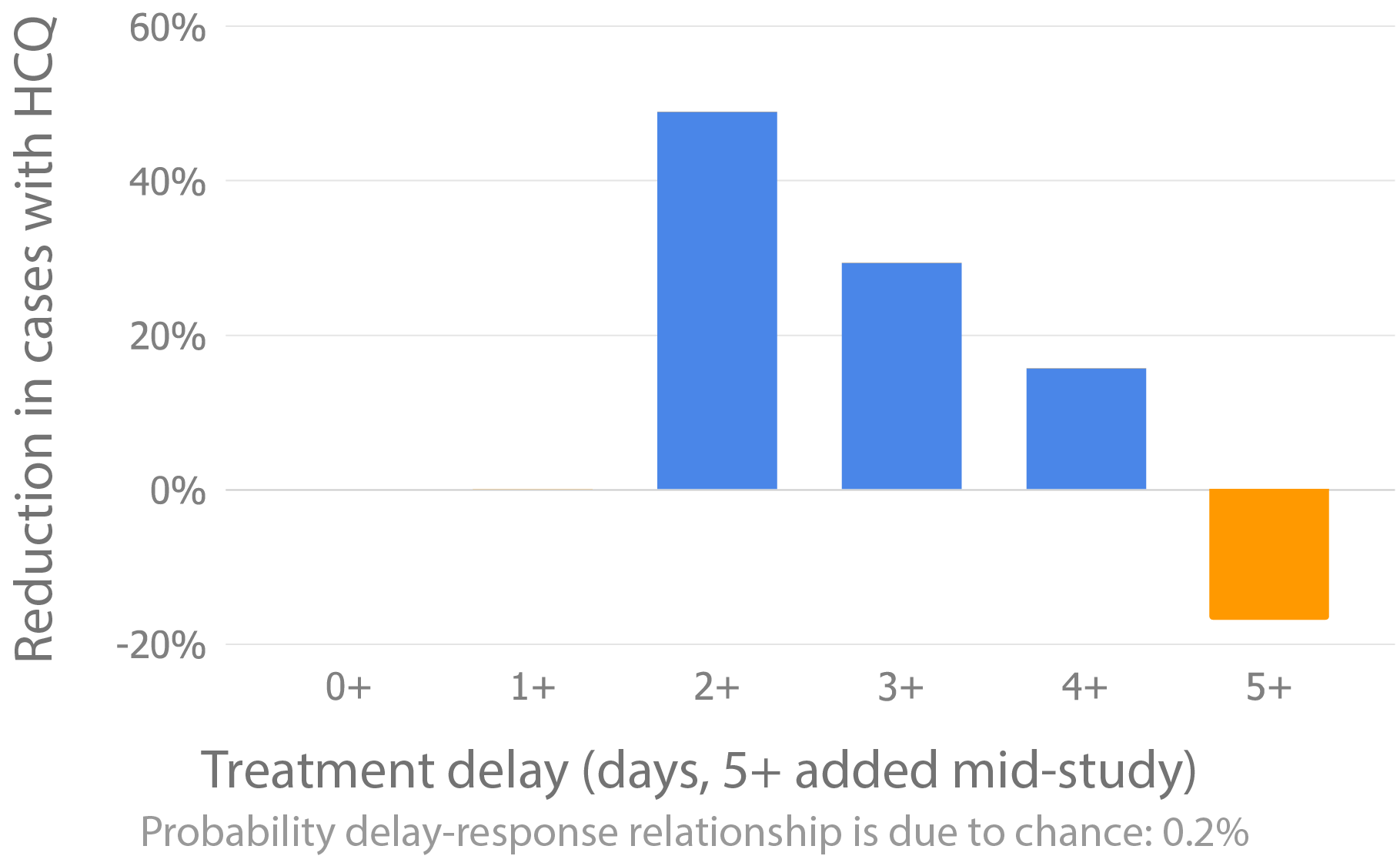
However, this statement is incorrect - cases were reduced, just without statistical significance - it's not possible to conclude there was no efficacy. Additionally, treatment was not within 4 days - there was up to 68 hours shipping delay as below.
COVID-19 cases were reduced by [49%, 29%, 16%] respectively when taken within ~[70, 94, 118] hours of exposure (including shipping delay). The treatment delay-response relationship is significant at p=0.002. For more detailed analysis, see8.
See also:9.
Authors compare with treatment with folic acid. Kaur et al. note that folic acid is predicted to bind to multiple SARS-CoV-2 proteins, folic acid levels are lower in COVID-19 patients with severe disease, folic acid supplementation may help with COVID-19 associated hypertension and hyperhomocystinemia, and differences in a folic acid-related enzyme could impact COVID-19 geographical severity variation.
Time of dosing was not recorded in these trials:11. See12, and Pullen et al.13, which shows shipping delay for these trials of 19 - 68 hours. With enrollment up to 4 days from exposure, this implies delivery 19 - 164 hours after exposure.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments14.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of case, 17.0% lower, RR 0.83, p = 0.35, treatment 49 of 414 (11.8%), control 58 of 407 (14.3%), NNT 41.
|
|
risk of case, 25.1% lower, RR 0.75, p = 0.22, treatment 32 of 414 (7.7%), control 42 of 407 (10.3%), NNT 39, probable COVID-19 cases.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
4.
researchgate.net, www.researchgate.net/publication/344369617_Hydroxychloroquine_as_Post-Exposure_Prophylaxis_for_Covid-19_Why_simple_data_analysis_can_lead_to_the_wrong_conclusions_from_well-designed_studies.
5.
blog.philbirnbaum.com, blog.philbirnbaum.com/2020/08/the-nejm-hydroxychloroquine-study-fails.html.
6.
longdom.org, www.longdom.org/open-access/hydroxychloroquine-and-interferons-for-the-prophylaxis-and-early-treatment-of-covid19current-clinical-advances.pdf.
Boulware et al., 3 Jun 2020, Randomized Controlled Trial, USA, peer-reviewed, 24 authors, study period 17 March, 2020 - 6 May, 2020, this trial compares with another treatment - results may be better when compared to placebo.
A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19
New England Journal of Medicine, doi:10.1056/nejmoa2016638
BACKGROUND Coronavirus disease 2019 (Covid-19) occurs after exposure to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). For persons who are exposed, the standard of care is observation and quarantine. Whether hydroxychloroquine can prevent symptomatic infection after SARS-CoV-2 exposure is unknown.
METHODS We conducted a randomized, double-blind, placebo-controlled trial across the United States and parts of Canada testing hydroxychloroquine as postexposure prophylaxis. We enrolled adults who had household or occupational exposure to someone with confirmed Covid-19 at a distance of less than 6 ft for more than 10 minutes while wearing neither a face mask nor an eye shield (high-risk exposure) or while wearing a face mask but no eye shield (moderate-risk exposure). Within 4 days after exposure, we randomly assigned participants to receive either placebo or hydroxychloroquine (800 mg once, followed by 600 mg in 6 to 8 hours, then 600 mg daily for 4 additional days). The primary outcome was the incidence of either laboratory-confirmed Covid-19 or illness compatible with Covid-19 within 14 days.
RESULTS We enrolled 821 asymptomatic participants. Overall, 87.6% of the participants (719 of 821) reported a high-risk exposure to a confirmed Covid-19 contact. The incidence of new illness compatible with Covid-19 did not differ significantly between participants receiving hydroxychloroquine (49 of 414 [11.8%]) and those receiving placebo (58 of 407 [14.3%]); the absolute difference was −2.4 percentage points (95% confidence interval, −7.0 to 2.2; P = 0.35). Side effects were more common with hydroxychloroquine than with placebo (40.1% vs. 16.8%), but no serious adverse reactions were reported.
CONCLUSIONS After high-risk or moderate-risk exposure to Covid-19, hydroxychloroquine did not prevent illness compatible with Covid-19 or confirmed infection when used as postexposure prophylaxis within 4 days after exposure. (Funded by David Baszucki and Jan Ellison Baszucki and others; ClinicalTrials.gov number, NCT04308668.
n engl j med nejm.org
8 T h e ne w e ngl a nd jou r na l o f m e dicine exposure prophylaxis would be effective in highrisk populations is a separate question, with trials ongoing. In order to end the pandemic, a reduction in community transmission is needed. Disclosure forms provided by the authors are available with the full text of this article at NEJM.org. A data sharing statement provided by the authors is available with the full text of this article at NEJM.org. We thank the participants who consented to participate in this randomized trial; the members of the data and safety monitoring board (Drs. George Thompson III, Andrej Spec, Tom Chiller, and Bozena Morawski) for their thoughtful, generous service; and Drs. Jakub Tolar, Alexis Turgeon, Brad Benson, Tim Schacker, and Peter Igarashi for institutional support. Dr. Boulware thanks Drs. Paul Bohjanen and Ed Janoff for their mentorship. * Values are through day 5, the date of the scheduled completion of the trial intervention. More than one side effect could occur. Ongoing side effects were reported by approximately 3% of the participants in the hydroxychloroquine group at days 10 and 14 and by less than 1% of those in the placebo group. There was no association between the occurrence of side effects and the incidence of Covid-19. Among participants in whom Covid-19 developed, 30.0% (30 of 100) reported a side effect, as compared with 28.2% (169 of 600) reporting a side effect in whom Covid-19 did not develop (P =..
DOI record:
{
"DOI": "10.1056/nejmoa2016638",
"ISSN": [
"0028-4793",
"1533-4406"
],
"URL": "http://dx.doi.org/10.1056/NEJMoa2016638",
"alternative-id": [
"10.1056/NEJMoa2016638"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-4715-0060",
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"authenticated-orcid": false,
"family": "Boulware",
"given": "David R.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"family": "Pullen",
"given": "Matthew F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"family": "Bangdiwala",
"given": "Ananta S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"family": "Pastick",
"given": "Katelyn A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"family": "Lofgren",
"given": "Sarah M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"family": "Okafor",
"given": "Elizabeth C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"family": "Skipper",
"given": "Caleb P.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"family": "Nascene",
"given": "Alanna A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5513-5509",
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"authenticated-orcid": false,
"family": "Nicol",
"given": "Melanie R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"family": "Abassi",
"given": "Mahsa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"family": "Engen",
"given": "Nicole W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"family": "Cheng",
"given": "Matthew P.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"family": "LaBar",
"given": "Derek",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"family": "Lother",
"given": "Sylvain A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"family": "MacKenzie",
"given": "Lauren J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"family": "Drobot",
"given": "Glen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"family": "Marten",
"given": "Nicole",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"family": "Zarychanski",
"given": "Ryan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"family": "Kelly",
"given": "Lauren E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"family": "Schwartz",
"given": "Ilan S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"family": "McDonald",
"given": "Emily G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"family": "Rajasingham",
"given": "Radha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"family": "Lee",
"given": "Todd C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Minnesota (D.R.B., M.F.P., A.S.B., K.A.P., S.M.L., E.C.O., C.P.S., A.A.N., M.R.N., M.A., N.W.E., R.R., K.H.H.) and M Health Fairview Investigational Drug Service Pharmacy (D.L.), Minneapolis; and the Research Institute of the McGill University Health Centre and the Clinical Practice Assessment Unit, Department of Medicine, McGill University, Montreal (M.P.C., E.G.M., T.C.L.), the Department of Internal Medicine, University of Manitoba (S.A.L., L.J.M., G.D., N.M., R.Z.), the..."
}
],
"family": "Hullsiek",
"given": "Kathy H.",
"sequence": "additional"
}
],
"container-title": "New England Journal of Medicine",
"container-title-short": "N Engl J Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
6,
3
]
],
"date-time": "2020-06-03T21:01:27Z",
"timestamp": 1591218087000
},
"deposited": {
"date-parts": [
[
2020,
12,
4
]
],
"date-time": "2020-12-04T14:19:27Z",
"timestamp": 1607091567000
},
"funder": [
{
"DOI": "10.13039/100007249",
"doi-asserted-by": "publisher",
"name": "University of Minnesota Foundation"
},
{
"name": "Jan and David Baszucki"
},
{
"name": "Alliance of Minnesota Chinese Organizations"
},
{
"name": "Minnesota Chinese Chamber of Commerce"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
5
]
],
"date-time": "2024-04-05T02:20:04Z",
"timestamp": 1712283604396
},
"is-referenced-by-count": 968,
"issue": "6",
"issued": {
"date-parts": [
[
2020,
8,
6
]
]
},
"journal-issue": {
"issue": "6",
"published-print": {
"date-parts": [
[
2020,
8,
6
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://www.nejmgroup.org/legal/terms-of-use.htm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
8,
6
]
],
"date-time": "2020-08-06T00:00:00Z",
"timestamp": 1596672000000
}
}
],
"link": [
{
"URL": "http://www.nejm.org/doi/pdf/10.1056/NEJMoa2016638",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "150",
"original-title": [],
"page": "517-525",
"prefix": "10.1056",
"published": {
"date-parts": [
[
2020,
8,
6
]
]
},
"published-print": {
"date-parts": [
[
2020,
8,
6
]
]
},
"publisher": "Massachusetts Medical Society",
"reference": [
{
"DOI": "10.1056/NEJMoa2001316",
"doi-asserted-by": "publisher",
"key": "r1"
},
{
"DOI": "10.1186/1743-422X-2-69",
"doi-asserted-by": "publisher",
"key": "r2"
},
{
"DOI": "10.1093/cid/ciaa237",
"doi-asserted-by": "publisher",
"key": "r3"
},
{
"DOI": "10.1038/s41421-020-0156-0",
"doi-asserted-by": "publisher",
"key": "r4"
},
{
"DOI": "10.1093/ofid/ofaa130",
"doi-asserted-by": "publisher",
"key": "r5"
},
{
"DOI": "10.1056/NEJMoa2012410",
"doi-asserted-by": "publisher",
"key": "r6"
},
{
"DOI": "10.1016/S0140-6736(20)31180-6",
"doi-asserted-by": "publisher",
"key": "r7"
},
{
"DOI": "10.1001/jama.2020.8630",
"doi-asserted-by": "publisher",
"key": "r8"
},
{
"DOI": "10.15585/mmwr.mm6909e1",
"doi-asserted-by": "publisher",
"key": "r9"
},
{
"DOI": "10.3201/eid2608.201274",
"doi-asserted-by": "publisher",
"key": "r10"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105988",
"doi-asserted-by": "publisher",
"key": "r11"
},
{
"DOI": "10.1007/s12630-020-01684-7",
"doi-asserted-by": "publisher",
"key": "r12"
},
{
"DOI": "10.1016/j.jbi.2019.103208",
"doi-asserted-by": "publisher",
"key": "r13"
},
{
"DOI": "10.1002/cpt.1874",
"doi-asserted-by": "publisher",
"key": "r14"
},
{
"DOI": "10.1056/NEJMoa2005412",
"doi-asserted-by": "publisher",
"key": "r16"
},
{
"DOI": "10.15585/mmwr.mm6915e6",
"doi-asserted-by": "publisher",
"key": "r17"
}
],
"reference-count": 16,
"references-count": 16,
"relation": {
"has-review": [
{
"asserted-by": "object",
"id": "10.3410/f.738066801.793579085",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.3410/f.738066801.793577585",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "http://www.nejm.org/doi/10.1056/NEJMoa2016638"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19",
"type": "journal-article",
"volume": "383"
}
