Early treatment with inhaled GM-CSF improves oxygenation and anti-viral immunity in COVID-19 induced lung injury – a randomized clinical trial
et al., Research Square, doi:10.21203/rs.3.rs-959220/v1, SARPAC, NCT04326920, Oct 2021
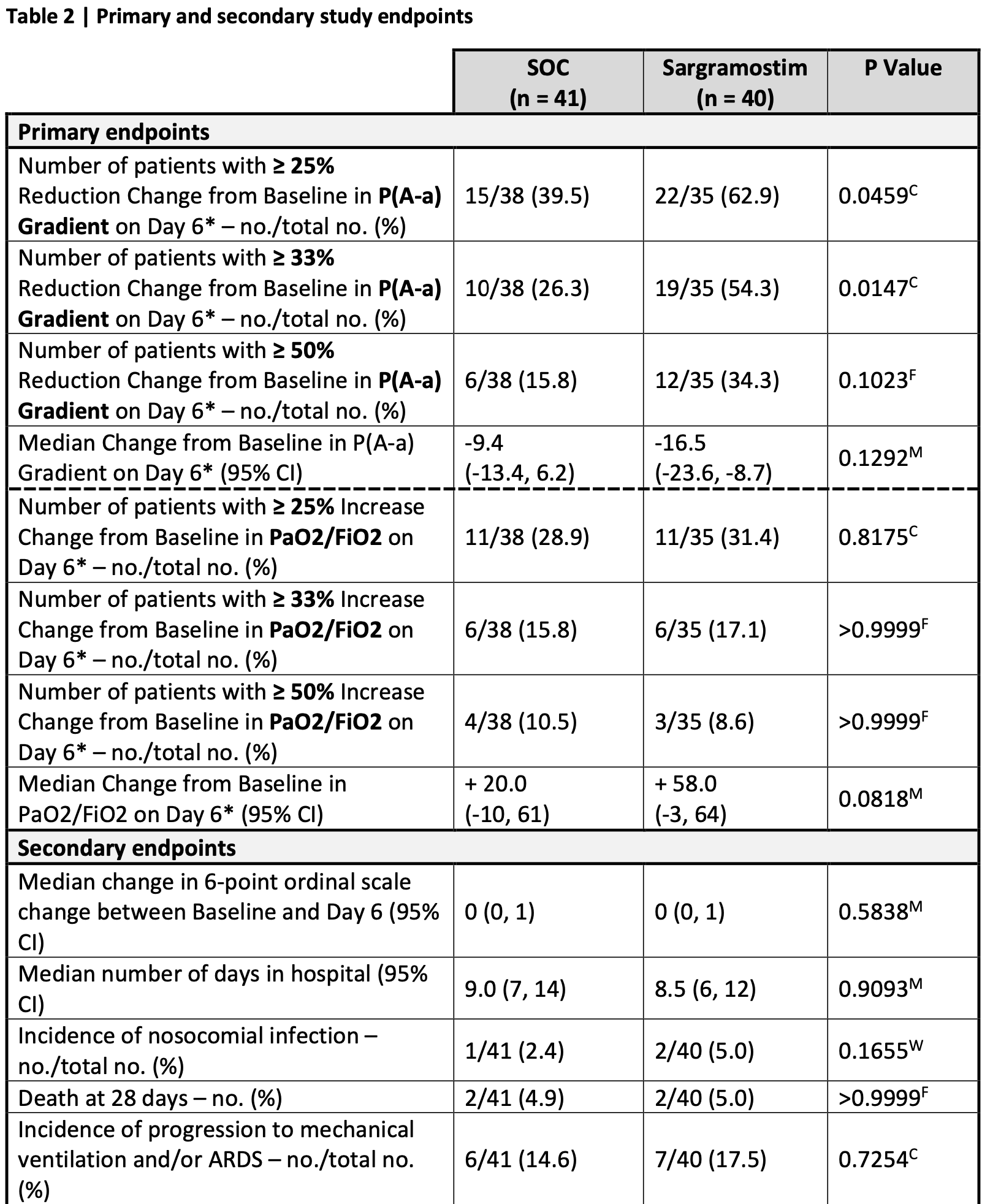
RCT 81 non-ventilated COVID-19 patients with hypoxemic respiratory failure showing improved oxygenation after 5 days of inhaled sargramostim (rhu-GM-CSF) compared to standard of care. More patients in the sargramostim group experienced at least 25% improvement in oxygenation. Sargramostim treatment also increased circulating class-switched B cells and effector SARS-CoV-2 specific CD8 T cells. There were no significant differences in mortality or clinical scores.
|
risk of death, 2.5% higher, RR 1.02, p = 1.00, treatment 2 of 40 (5.0%), control 2 of 41 (4.9%).
|
|
risk of mechanical ventilation, 19.6% higher, RR 1.20, p = 0.77, treatment 7 of 40 (17.5%), control 6 of 41 (14.6%).
|
|
relative clinical score improvement, 10.0% worse, RR 1.10, p = 0.77, treatment mean 2.0 (±3.1) n=40, control mean 2.2 (±3.0) n=41, day 6.
|
|
relative NEWS2 improvement, 33.3% better, RR 0.67, p = 0.77, treatment mean 0.6 (±3.1) n=40, control mean 0.4 (±3.0) n=41, day 6.
|
|
relative SOFA improvement, 20.0% better, RR 0.80, p = 0.74, treatment mean 0.5 (±1.5) n=40, control mean 0.4 (±1.2) n=41, day 6.
|
|
relative change in P(A-a) gradient, 43.0% better, RR 0.57, p = 0.13, treatment 40, control 41, day 6.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Bosteels et al., 13 Oct 2021, Randomized Controlled Trial, Belgium, preprint, median age 60.0, 31 authors, trial NCT04326920 (history) (SARPAC).
Contact: bart.lambrecht@ugent.be, cedric.bosteels@ugent.be.
Early treatment with inhaled GM-CSF improves oxygenation and anti-viral immunity in COVID-19 induced lung injury – a randomized clinical trial
doi:10.21203/rs.3.rs-959220/v1
Granulocyte-macrophage colony-stimulating factor (GM-CSF) instructs monocytes to differentiate into alveolar macrophages (AM) that preserve lung homeostasis. By comparing AM development in mouse and human, we discovered that COVID-19 patients showed marked defects in GM-CSF-dependent AM instruction. The multi-center, open-label, randomized, controlled SARPAC-trial evaluated the efficacy and safety of 5 days of inhalation of rhu-GM-CSF (sargramostim, Leukine®) in 81 non-ventilated patients with COVID-19 and hypoxemic respiratory failure identified by PaO2/FiO2 ratio < 350mmHg. At day 6, more patients in the sargramostim group experienced at least 25% improvement in oxygenation compared with the standard of care group. Higher numbers of circulating class-switched B cells and effector virus-specific CD8 lymphocytes were found in the sargramostim group. Treatment adverse events, including signs of cytokine storm, were not different between active and control group. This proof-of-concept study demonstrates the feasibility and safety of inhaled GM-CSF in restoring alveolar gas exchange, while simultaneously boosting anti-COVID-19 immunity. ClinicalTrials.gov (NCT04326920).
Complement components were measured in cell free plasma. C5a was measured using customizable enzyme immunoassay multiplex kits (MicroVue Complement Multiplex, Quidel; A905s), according to manufacturer's instructions. Data were acquired on a Q-View Imager LS, using the Q-View Software 3.11.
Immunoglobulin ELISA SARS-CoV2 antibodies on stored serum samples of included patients were analyzed with antigen-coated ELISA kits (EUROIMMUN AG) for anti-spike 1 (S1) IgA (EI 2606-9601 A) and IgG (EI 2606-9601 G) and anti-nucleocapsid protein (NCP) IgG (EI 2606-9601-2 G), according to manufacturer's protocol.
Trial oversight and role of the funder The trial was approved by the competent authorities and the Ethical Committee of Ghent University Hospital, and the trial was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. Bart N. Lambrecht designed the trial and was the coordinating investigator. An independent data safety monitoring board monitored participant safety. Every patient or their legal representative provided informed consent for participation. All investigators take responsibility for the integrity of the trial and the publication. The first authors wrote the first draft of the manuscript. All authors made the decision to submit the manuscript for publication and vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol.
Competing interests
Supplementary Files This is a list of..
References
Aegerter, Kulikauskaite, Crotta, Influenza-induced monocyte-derived alveolar macrophages confer prolonged antibacterial protection, Nat Immunol, doi:10.1038/s41590-019-0568-x
Bittmann, Dose, Baretton, Cellular chimerism of the lung after transplantation. An interphase cytogenetic study, Am J Clin Pathol
Bost, Giladi, Liu, Host-Viral Infection Maps Reveal Signatures of Severe COVID-19 Patients, Cell, doi:10.1016/j.cell.2020.05.006
Bost, Sanctis, Cane, Deciphering the state of immune silence in fatal COVID-19 patients, Nat Commun, doi:10.1038/s41467-021-21702-6
Bosteels, Maes, Van Damme, Sargramostim to treat patients with acute hypoxic respiratory failure due to COVID-19 (SARPAC): A structured summary of a study protocol for a randomised controlled trial, Trials, doi:10.1186/s13063-020-04451-7
Byrne, Powell, Sullivan, Dynamics of human monocytes and airway macrophages during healthy aging and after transplant, J Exp Med. Mar, doi:10.1084/jem.20191236
Carvelli, Demaria, Vely, Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis, Nature, doi:10.1038/s41586-020-2600-6
Chua, Lukassen, Trump, COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis, Nature biotechnology, doi:10.1038/s41587-020-0602-4
Cremer, Abbate, Hudock, Mavrilimumab in patients with severe COVID-19 pneumonia and systemic hyperinflammation (MASH-COVID): an investigator initiated, multicentre, double-blind, randomised, placebo-controlled trial, Lancet Rheumatol, doi:10.1016/S2665-9913(21)00070-9
De Alessandris, Ferguson, Dodd, Neutrophil GM-CSF receptor dynamics in acute lung injury, J Leukoc Biol, doi:10.1002/JLB.3MA0918-347R
Dorward, Russell, Um, Tissue-Specific Immunopathology in Fatal COVID-19, Am J Respir Crit Care Med, doi:10.1164/rccm.202008-3265OC
Gautier, Shay, Miller, Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages, Nat Immunol, doi:10.1038/ni.2419
Gayoso, Steier, Lopez, Joint probabilistic modeling of single-cell multiomic data with totalVI, Nat Methods, doi:10.1038/s41592-020-01050-x
Gowda, Klocke, Variability of indices of hypoxemia in adult respiratory distress syndrome, Crit Care Med, doi:10.1097/00003246-199701000-00010
Grant, Morales-Nebreda, Markov, Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia, Nature, doi:10.1038/s41586-020-03148-w
Grifoni, Sidney, Vita, SARS-CoV-2 human T cell epitopes: Adaptive immune response against COVID-19, Cell Host Microbe, doi:10.1016/j.chom.2021.05.010
Gschwend, Sherman, Ridder, Alveolar macrophages rely on GM-CSF from alveolar epithelial type 2 cells before and after birth, J Exp Med, doi:10.1084/jem.20210745
Gu, Tyagi, Jain, Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation, Nat Rev Cardiol, doi:10.1038/s41569-020-00469-1
Guan, Ni, Hu, Clinical Characteristics of Coronavirus Disease 2019 in China, N Engl J Med, doi:10.1056/NEJMoa2002032
Guilliams, Kleer, Henri, Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF, J Exp Med, doi:10.1084/jem.20131199jem.20131199
Guimaraes, Quirk, Furtado, Tofacitinib in Patients Hospitalized with Covid-19 Pneumonia, N Engl J Med, doi:10.1056/NEJMoa2101643
Hao, Hao, Andersen-Nissen, Integrated analysis of multimodal single-cell data, Cell. Jun, doi:10.1016/j.cell.2021.04.048
Harris, Taylor, Thielke, Payne, Gonzalez et al., Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform, doi:10.1016/j.jbi.2008.08.010297.00(242.00-319.50)291.50(251.50-329.00)295.00(248.00-328
Hashimoto, Chow, Noizat, Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes, Immunity, doi:10.1016/j.immuni.2013.04.004S1074-7613(13)00157-X
Herold, Hoegner, Vadasz, Inhaled granulocyte/macrophage colonystimulating factor as treatment of pneumonia-associated acute respiratory distress syndrome, Am J Respir Crit Care Med, doi:10.1164/rccm.201311-2041LE
Huang, Barnes, Feng, GM-CSF in the lung protects against lethal influenza infection, Am J Respir Crit Care Med, doi:10.1164/rccm.201012-2036OC
Huffman, Hull, Dranoff, Mulligan, Whitsett, Pulmonary epithelial cell expression of GM-CSF corrects the alveolar proteinosis in GM-CSF-deficient mice, J Clin Invest, doi:10.1172/JCI118461
Hussell, Bell, Alveolar macrophages: plasticity in a tissue-specific context, Nat Rev Immunol, doi:10.1038/nri3600
Kyriazopoulou, Poulakou, Milionis, Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial, Nat Med, doi:10.1038/s41591-021-01499-z
Lang, Lee, Teijaro, Becher, Hamilton, GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches, Nat Rev Immunol, doi:10.1038/s41577-020-0357-7
Liao, Liu, Yuan, Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19, Nat Med. Jun, doi:10.1038/s41591-020-0901-9
Liao, Wang, Chen, Down-regulation of granulocyte-macrophage colony-stimulating factor by 3C-like proteinase in transfected A549 human lung carcinoma cells, BMC Immunol, doi:10.1186/1471-2172-12-16
Lucas, Wong, Klein, Longitudinal analyses reveal immunological misfiring in severe COVID-19, Nature, doi:10.1038/s41586-020-2588-y
Ma, Kulkarni, Increased complement activation is a distinctive feature of severe SARS-CoV-2 infection, Sci Immunol, doi:10.1126/sciimmunol.abh2259
Machiels, Dourcy, Xiao, A gammaherpesvirus provides protection against allergic asthma by inducing the replacement of resident alveolar macrophages with regulatory monocytes, Nat Immunol, doi:10.1038/ni.3857
Matute-Bello, Liles, Radella, Modulation of neutrophil apoptosis by granulocyte colony-stimulating factor and granulocyte/macrophage colony-stimulating factor during the course of acute respiratory distress syndrome, Crit Care Med, doi:10.1097/00003246-200001000-00001
Mehta, Mcauley, Brown, COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet, doi:10.1016/S0140-6736(20)30628-0
Mehta, Porter, Manson, Therapeutic blockade of granulocyte macrophage colony-stimulating factor in COVID-19-associated hyperinflammation: challenges and opportunities, Lancet Respir Med, doi:10.1016/S2213-2600(20)30267-8
Merad, Martin, Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages, Nat Rev Immunol, doi:10.1038/s41577-020-0331-4
Morrissey, Geller, Hu, A specific low-density neutrophil population correlates with hypercoagulation and disease severity in hospitalized COVID-19 patients, JCI Insight, doi:10.1172/jci.insight.148435
Nayak, Zhou, Xu, Long-Term Persistence of Donor Alveolar Macrophages in Human Lung Transplant Recipients That Influences Donor-Specific Immune Responses, Am J Transplant, doi:10.1111/ajt.13819
Netea, Rovina, Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure, Cell Host Microbe, doi:10.1016/j.chom.2020.04.009
Neupane, Willson, Chojnacki, Patrolling Alveolar Macrophages Conceal Bacteria from the Immune System to Maintain Homeostasis, Cell. Oct, doi:10.1016/j.cell.2020.08.020
Overgaard, Schlingmann, White, The relative balance of GM-CSF and TGF-beta1 regulates lung epithelial barrier function, Am J Physiol Lung Cell Mol Physiol, doi:10.1152/ajplung.00042.2014
Paine, Standiford, Dechert, A randomized trial of recombinant human granulocyte-macrophage colony stimulating factor for patients with acute lung injury, Crit Care Med, doi:10.1097/CCM.0b013e31822d7bf0
Presneill, Harris, Stewart, Cade, Wilson, A randomized phase II trial of granulocyte-macrophage colony-stimulating factor therapy in severe sepsis with respiratory dysfunction, Am J Respir Crit Care Med, doi:10.1164/rccm.2009005
Rosler, Herold, Lung epithelial GM-CSF improves host defense function and epithelial repair in influenza virus pneumonia-a new therapeutic strategy?, Mol Cell Pediatr, doi:10.1186/s40348-016-0055-5
Sacchi, Grassi, Bordoni, Early expansion of myeloid-derived suppressor cells inhibits SARS-CoV-2 specific T-cell response and may predict fatal COVID-19 outcome, Cell death & disease, doi:10.1038/s41419-020-03125-1
Schneider, Nobs, Kurrer, Rehrauer, Thiele et al., Induction of the nuclear receptor PPAR-gamma by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages, Nat Immunol, doi:10.1038/ni.3005
Stephenson, Reynolds, Botting, Single-cell multi-omics analysis of the immune response in COVID-19, Nat Med, doi:10.1038/s41591-021-01329-2
Sterner, Sakemura, Cox, GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts, Blood, doi:10.1182/blood-2018-10-881722
Stoeckius, Hafemeister, Stephenson, Simultaneous epitope and transcriptome measurement in single cells, Nat Methods, doi:10.1038/nmeth.4380
Street, Risso, Fletcher, Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics, BMC Genomics, doi:10.1186/s12864-018-4772-0
Sturrock, Seedahmed, Mir-Kasimov, Boltax, Mcmanus et al., GM-CSF provides autocrine protection for murine alveolar epithelial cells from oxidant-induced mitochondrial injury, Am J Physiol Lung Cell Mol Physiol, doi:10.1152/ajplung.00276.2011
Sturrock, Vollbrecht, Mir-Kasimov, Mcmanus, Wilcoxen et al., Mechanisms of suppression of alveolar epithelial cell GM-CSF expression in the setting of hyperoxic stress, Am J Physiol Lung Cell Mol Physiol, doi:10.1152/ajplung.00161.2009
Subramaniam, Barnes, Fletcher, Protecting against post-influenza bacterial pneumonia by increasing phagocyte recruitment and ROS production, J Infect Dis, doi:10.1093/infdis/jit830
Suzuki, Sakagami, Rubin, Familial pulmonary alveolar proteinosis caused by mutations in CSF2RA, J Exp Med, doi:10.1084/jem.20080990
Tazawa, Hamano, Arai, Granulocyte-macrophage colony-stimulating factor and lung immunity in pulmonary alveolar proteinosis, Am J Respir Crit Care Med, doi:10.1164/rccm.200406-716OC
Tazawa, Inoue, Arai, Duration of benefit in patients with autoimmune pulmonary alveolar proteinosis after inhaled granulocyte-macrophage colony-stimulating factor therapy, Chest, doi:10.1378/chest.13-0603
Tazawa, Trapnell, Inoue, Inhaled granulocyte/macrophage-colony stimulating factor as therapy for pulmonary alveolar proteinosis, Am J Respir Crit Care Med, doi:10.1164/rccm.200906-0978OC
Tazawa, Ueda, Abe, Inhaled GM-CSF for Pulmonary Alveolar Proteinosis, N Engl J Med, doi:10.1056/NEJMoa1816216
Temesgen, Burger, Baker, Lenzilumab Efficacy and Safety in Newly Hospitalized Covid-19 Subjects: Results from the Live-Air Phase 3 Randomized Double-Blind Placebo-Controlled Trial, medRxiv, doi:10.1101/2021.05.01.21256470
Thwaites, Sevilla Uruchurtu, Siggins, Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe COVID-19, Sci Immunol, doi:10.1126/sciimmunol.abg9873
Tobin, Jubran, Laghi, P aO2 /F IO2 ratio: the mismeasure of oxygenation in COVID-19, Eur Respir J. Mar, doi:10.1183/13993003.00274-2021
Unkel, Hoegner, Clausen, Alveolar epithelial cells orchestrate DC function in murine viral pneumonia, J Clin Invest, doi:10.1172/JCI62139
Vabret, Britton, Gruber, Immunology of COVID-19: Current State of the Science, Immunity, doi:10.1016/j.immuni.2020.05.002
Valle, Kim-Schulze, Huang, An inflammatory cytokine signature predicts COVID-19 severity and survival, Nat Med, doi:10.1038/s41591-020-1051-9
Van De Laar, Saelens, Prijck, Yolk Sac Macrophages, Fetal Liver, and Adult Monocytes Can Colonize an Empty Niche and Develop into Functional Tissue-Resident Macrophages, Immunity, doi:10.1016/j.immuni.2016.02.017
Vanderbeke, Van Mol, Herck, Monocyte-driven atypical cytokine storm and aberrant neutrophil activation as key mediators of COVID-19 disease severity, Nat Commun, doi:10.1038/s41467-021-24360-w
Wauters, Van Mol, Garg, Discriminating mild from critical COVID-19 by innate and adaptive immune single-cell profiling of bronchoalveolar lavages, Cell Res, doi:10.1038/s41422-020-00455-9
Weiskopf, Schmitz, Raadsen, Phenotype and kinetics of SARS-CoV-2specific T cells in COVID-19 patients with acute respiratory distress syndrome, Sci Immunol, doi:10.1126/sciimmunol.abd2071
Westphalen, Gusarova, Islam, Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity, Nature, doi:10.1038/nature12902
Whoreactgroup, Shankar-Hari, Vale, Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Metaanalysis, JAMA, doi:10.1001/jama.2021.11330
Xu, Shi, Wang, Pathological findings of COVID-19 associated with acute respiratory distress syndrome, Lancet Respir Med, doi:10.1016/S2213-2600(20)30076-X
Yona, Kim, Wolf, Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis, Immunity, doi:10.1016/j.immuni.2012.12.001S1074-7613(12)00548-1
Yoshida, Ikegami, Reed, Chroneos, Whitsett, GM-CSF regulates protein and lipid catabolism by alveolar macrophages, Am J Physiol Lung Cell Mol Physiol, doi:10.1152/ajplung.2001.280.3.L379
Zhao, Kilian, Turner, Clonal expansion and activation of tissue-resident memory-like Th17 cells expressing GM-CSF in the lungs of severe COVID-19 patients, Sci Immunol, doi:10.1126/sciimmunol.abf6692
Zhou, To, Wong, Acute SARS-CoV-2 Infection Impairs Dendritic Cell and T Cell Responses, Immunity, doi:10.1016/j.immuni.2020.07.026
DOI record:
{
"DOI": "10.21203/rs.3.rs-959220/v1",
"URL": "http://dx.doi.org/10.21203/rs.3.rs-959220/v1",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>Granulocyte-macrophage colony-stimulating factor (GM-CSF) instructs monocytes to differentiate into alveolar macrophages (AM) that preserve lung homeostasis. By comparing AM development in mouse and human, we discovered that COVID-19 patients showed marked defects in GM-CSF-dependent AM instruction. The multi-center, open-label, randomized, controlled SARPAC-trial evaluated the efficacy and safety of 5 days of inhalation of rhu-GM-CSF (sargramostim, Leukine®) in 81 non-ventilated patients with COVID-19 and hypoxemic respiratory failure identified by PaO2/FiO2 ratio < 350mmHg. At day 6, more patients in the sargramostim group experienced at least 25% improvement in oxygenation compared with the standard of care group. Higher numbers of circulating class-switched B cells and effector virus-specific CD8 lymphocytes were found in the sargramostim group. Treatment adverse events, including signs of cytokine storm, were not different between active and control group. This proof-of-concept study demonstrates the feasibility and safety of inhaled GM-CSF in restoring alveolar gas exchange, while simultaneously boosting anti-COVID-19 immunity. ClinicalTrials.gov (NCT04326920).</jats:p>",
"accepted": {
"date-parts": [
[
2021,
10,
7
]
]
},
"author": [
{
"affiliation": [
{
"name": "VIB-Ghent University"
}
],
"family": "Bosteels",
"given": "Cedric",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-4379-1047",
"affiliation": [
{
"name": "VIB-Ghent University"
}
],
"authenticated-orcid": false,
"family": "Damme",
"given": "Karel Van",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "VIB-Ghent University"
}
],
"family": "De Leeuw",
"given": "Elisabeth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "VIB-Ghent University"
}
],
"family": "Declercq",
"given": "Jozefien",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "VIB-Ghent University"
}
],
"family": "Maes",
"given": "Bastiaan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "VIB-Ghent University"
}
],
"family": "Bosteels",
"given": "Victor",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ghent University"
}
],
"family": "Hoste",
"given": "Levi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ghent University"
}
],
"family": "Naesens",
"given": "Leslie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "VIB-Ghent University"
}
],
"family": "Debeuf",
"given": "Nincy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "VIB-Ghent University"
}
],
"family": "Deckers",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Center for Infectious Disease, La Jolla Institute for Immunology, La Jolla, CA92037, USA"
}
],
"family": "Weiskopf",
"given": "Daniela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "La Jolla Institute for Allergy and Immunology"
}
],
"family": "Sette",
"given": "Alessandro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ghent University"
}
],
"family": "Weygaerde",
"given": "Yannick Vande",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ghent University"
}
],
"family": "Malfait",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AZ Sint-Jan Hospital"
}
],
"family": "Vandecasteele",
"given": "Stefaan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AZ Delta General Hospital"
}
],
"family": "Demedts",
"given": "Ingel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "ZNA General Hospital"
}
],
"family": "Slabbynck",
"given": "Hans",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Universitair Ziekenhuis Brussel"
}
],
"family": "Allard",
"given": "Sabine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ghent University"
}
],
"family": "Depuydt",
"given": "Pieter",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7242-0747",
"affiliation": [
{
"name": "Ghent University"
}
],
"authenticated-orcid": false,
"family": "Braeckel",
"given": "Eva Van",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ghent University"
}
],
"family": "De Clercq",
"given": "Jozefien",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "VIB-Ghent University"
}
],
"family": "Martens",
"given": "Liesbet",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "VIB-Ghent University"
}
],
"family": "Dupont",
"given": "Sam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "VIB-Ghent University"
}
],
"family": "Seurinck",
"given": "Ruth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Cancer Research Institute Ghent (CRIG)"
}
],
"family": "Vandamme",
"given": "Niels",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ghent University Hospital"
}
],
"family": "Haerynck",
"given": "Filomeen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Partner therapeutics"
}
],
"family": "Roychowdhury",
"given": "Debasish",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8600-1631",
"affiliation": [
{
"name": "HIV Cure Research Center, Ghent University Hospital"
}
],
"authenticated-orcid": false,
"family": "Vandekerckhove",
"given": "Linos",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "VIB-Ghent University"
}
],
"family": "Guilliams",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "VIB-Ghent University"
}
],
"family": "Tavernier",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "VIB Center for Inflammation Research"
}
],
"family": "Lambrecht",
"given": "Bart",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
10,
13
]
],
"date-time": "2021-10-13T22:44:14Z",
"timestamp": 1634165054000
},
"deposited": {
"date-parts": [
[
2021,
11,
4
]
],
"date-time": "2021-11-04T18:00:40Z",
"timestamp": 1636048840000
},
"group-title": "In Review",
"indexed": {
"date-parts": [
[
2024,
5,
27
]
],
"date-time": "2024-05-27T05:03:59Z",
"timestamp": 1716786239119
},
"institution": [
{
"name": "Research Square"
}
],
"is-referenced-by-count": 2,
"issued": {
"date-parts": [
[
2021,
10,
13
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
13
]
],
"date-time": "2021-10-13T00:00:00Z",
"timestamp": 1634083200000
}
}
],
"link": [
{
"URL": "https://www.researchsquare.com/article/rs-959220/v1",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.researchsquare.com/article/rs-959220/v1.html",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "8761",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
10,
13
]
]
},
"prefix": "10.21203",
"published": {
"date-parts": [
[
2021,
10,
13
]
]
},
"publisher": "Research Square Platform LLC",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.researchsquare.com/article/rs-959220/v1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "Early treatment with inhaled GM-CSF improves oxygenation and anti-viral immunity in COVID-19 induced lung injury – a randomized clinical trial",
"type": "posted-content"
}