Efficacy and Safety of Ensovibep for Adults Hospitalized With COVID-19
et al., Annals of Internal Medicine, doi:10.7326/M22-1503, ACTIV-3/TICO, NCT04501978, Aug 2022
RCT 485 hospitalized patients showing no significant differences with ensovibep treatment. Intravenous ensovibep, 600mg. Long-term results are reported in Mourad et al.
|
risk of death, 28.0% lower, HR 0.72, p = 0.16, treatment 33 of 247 (13.4%), control 43 of 238 (18.1%), NNT 21, Cox proportional hazards, day 540.
|
|
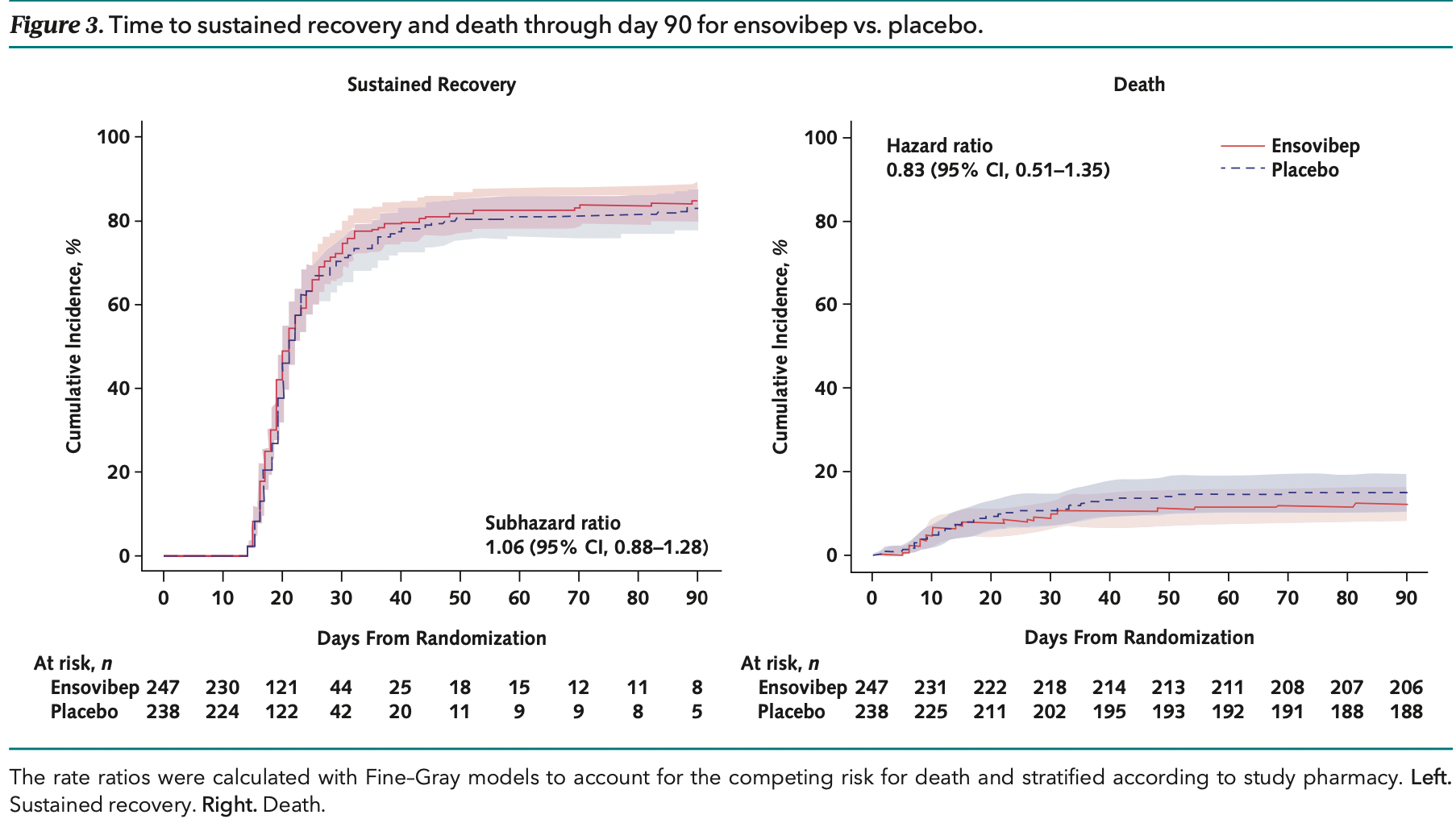
risk of death, 17.0% lower, HR 0.83, p = 0.46, treatment 30 of 247 (12.1%), control 35 of 238 (14.7%), NNT 39, Kaplan-Meier, day 90.
|
|
risk of death/hospitalization, 11.0% lower, HR 0.89, p = 0.47, treatment 80 of 247 (32.4%), control 85 of 238 (35.7%), NNT 30, Cox proportional hazards, day 540.
|
|
risk of no recovery, 5.7% lower, HR 0.94, p = 0.55, treatment 44 of 247 (17.8%), control 48 of 238 (20.2%), NNT 42, adjusted per study, inverted to make HR<1 favor treatment.
|
|
risk of no recovery, 7.5% higher, HR 1.08, p = 0.68, treatment 247, control 238, adjusted per study, inverted to make HR<1 favor treatment, pulmonary ordinal outcome, day 5.
|
|
risk of no hospital discharge, 6.5% lower, HR 0.93, p = 0.46, treatment 28 of 247 (11.3%), control 34 of 238 (14.3%), adjusted per study, inverted to make HR<1 favor treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Barkauskas et al., 9 Aug 2022, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, peer-reviewed, 80 authors, study period 11 June, 2021 - 15 November, 2021, average treatment delay 8.0 days, trial NCT04501978 (history) (ACTIV-3/TICO).
Efficacy and Safety of Ensovibep for Adults Hospitalized With COVID-19
Annals of Internal Medicine, doi:10.7326/m22-1503
Background: Ensovibep (MP0420) is a designed ankyrin repeat protein, a novel class of engineered proteins, under investigation as a treatment of SARS-CoV-2 infection. Objective: To investigate if ensovibep, in addition to remdesivir and other standard care, improves clinical outcomes among patients hospitalized with COVID-19 compared with standard care alone.
Author contributions are available at Annals.org.
References
Accardi, Shaw-Saliba, Phd; Robin, Dewar, None, PhD
Adit, Ginde, Md, Mph, None
Almasri, Md, None
Atri, Barkauskas, Brown, Chandra, Chang et al., Ensovibep for Adults Hospitalized With COVID-19 ORIGINAL RESEARCH Annals.org Author Contributions: Conception and design
Atri, Mph, None
Babiker, Phd, Victoria, Davey, Phd et al., None
Badrock, Bsc, None
Baduashvili, Md, None
Barmparessou, Md, None
Bednarska, Md, None
Beigel, Tomashek, Dodd, ACTT-1 Study Group Members. Remdesivir for the treatment of Covid-19 -final report, N Engl J Med, doi:10.1056/NEJMoa2007764
Binz, Amstutz, Kohl, High-affinity binders selected from designed ankyrin repeat protein libraries, Nat Biotechnol
Braun, Md; Huldrych, Günthard, Ramachandruni, None
Chang, Md, None
Chen, Md, None
Chen, Nirula, Heller, BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2029849
Cohen, Nirula, Mulligan, 2 Investigators. Effect of bamlanivimab vs placebo on incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities: a randomized clinical trial, JAMA, doi:10.1001/jama.2021.8828
Davey Rt, Fernández-Cruz, Markowitz, Study Group. Anti-influenza hyperimmune intravenous immunoglobulin for adults with influenza A or B infection (FLU-IVIG): a double-blind, randomised, placebo-controlled trial, Lancet Respir Med, doi:10.1016/S1473-3099(20)30483-7
Dougan, Nirula, Azizad, BLAZE-1 Investigators. Bamlanivimab plus etesevimab in mild or moderate Covid-19, N Engl J Med, doi:10.1056/NEJMoa2102685
Gerry, None
Grandits, Ms; Nilima, Mosaly, Md, None
Grund, Neaton, Phillips, Reilly, Shehadeh et al., Collection and assembly of data
Grund, None
Gupta, Gonzalez-Rojas, Juarez, Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N Engl J Med, doi:10.1056/NEJMoa2107934
Hatlen, Md, None
Hien, Nguyen, Md, None
Highbarger, Tauseef Rehman, None
Hudson, Ba, None
Hughes, Md; Sanjay, Bhagani, Md, None
Hui, Bbio, Leither, Do, None
Kaczynski, Bsc, Evangelia, Mylona, None, MSc
Kan, Md, None
Kelleher, Mbbs, Phd, Bsc, Nagy-Agren et al., None
Kidega, Mbchb, Mmed, None
Kim, Md, None
Kimuli, Mbchb, Mmed, None
Kirk, Knowlton, Md; Mamta, Jain, Md et al., None
Konstantinos, Syrigos, Md, None
Kumarasamy, Abrishamian, Bonten, Interim results from the randomised, controlled EMPATHY phase 2/3 study evaluating ensovibep, a DARPin therapeutic, in patients with mildto-moderate COVID-19
Legenne, Md, Chandra, Mbbs, Lane et al., None
Looney, Md, None
Lundgren, Drs, Barkauskas, Mylonakis, and Young contributed equally to this work
Lundgren, Grund, Barkauskas, ACTIV-3/TICO LY-CoV555 Study Group. A neutralizing monoclonal antibody for hospitalized patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2033130
Lundgren, Grund, Barkauskas, Study Group. Responses to a neutralizing monoclonal antibody for hospitalized patients with COVID-19 according to baseline antibody and antigen levels. A randomized controlled trial, Ann Intern Med, doi:10.7326/M21-3507
Lutaakome, Mbchb, Mph, None
Lye, Mbbs, None
Marines-Price, Phd, Osuji, Rn, Bsn et al., None
Matthay, Md, None
Matthews, Mbchb, Phd, Taylor Thompson, None
Menon, Md, None
Montgomery, Hobbs, Padilla, Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial, Lancet Respir Med, doi:10.1016/S2213-2600(22)00180-1
Murray, Babiker, Baker, Design and implementation of an international, multi-arm, multi-stage platform master protocol for trials of novel SARS-CoV-2 antiviral agents: Therapeutics for Inpatients with COVID-19 (TICO/ACTIV-3), Clin Trials, doi:10.1177/17407745211049829
Mylonakis, Md, Poulakou, Md, None
O'brien, Forleo-Neto, Sarkar, COVID-19 Phase 3 Prevention Trial Team. Effect of subcutaneous casirivimab and imdevimab antibody combination vs placebo on development of symptomatic COVID-19 in early asymptomatic SARS-CoV-2 infection: a randomized clinical trial, JAMA, doi:10.1001/jama.2021.24939
Overcash, Kalomenidis, Md, None
Paredes, Md, None
Pett, Md, None
Phillips, Phd, Daniel, Murray, Phd et al., None
Protopapas, Md, Koulouris, Md, None
Rapti, Md, None
Rapti, Robinson, Sandkovsky, Shehadeh, Siegel et al., Critical revision for important intellectual content
Robinson, Md, None
Rodger, Md, None
Rogers, Shehadeh, None, MSc
Rothenberger, Hurdiss, Walser, Ensovibep, a novel trispecific DARPin candidate that protects against SARS-CoV-2 variants. bioRxiv, doi:10.1101/2021.02.03.429164
Sandkovsky, Md, Ms; Robert, Gottlieb, Nnakelu et al., None
Schechner, Md, None
Sebudde, Mbchb, None
Sharma, Ms; Cavan, Reilly, None
Stumpp, Dawson, Binz, Beyond antibodies: the DARPin drug platform, BioDrugs, doi:10.1007/s40259-020-00429-8
Sánchez, Ms; Amy, Weintrob, Md, None
Takashita, Kinoshita, Yamayoshi, Efficacy of antibodies and antiviral drugs against Covid-19 omicron variant
Teitelbaum, Md, Ms, None
Torbati, Kiweewa, Mbchb, Mmed, Mph, None
Touloumi, Phd, Samuel, Brown, Md; Wesley et al., None
Trautner, Md, None
Vall Ee, Pharmd, Crew, Pharmd, Mph, None
Vasudeva, Md, None
Vekstein, Md, None
Ven Natarajan, Phd; Sylvain Laverdure, None
Vogel, Rn, Bsn, None
Walser, Rothenberger, Hurdiss, Highly potent anti-SARS-CoV-2 multivalent DARPin therapeutic candidates. bioRxiv, doi:10.1101/2020.08.25.256339
Waters, Md, None
Weinreich, Sivapalasingam, Norton, Trial Investigators. REGEN-COV antibody combination and outcomes in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2108163
Weinreich, Sivapalasingam, Norton, Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2035002
Young, Md, Phd, David, Vock et al., None
Zepeda, Md, None
DOI record:
{
"DOI": "10.7326/m22-1503",
"ISSN": [
"0003-4819",
"1539-3704"
],
"URL": "http://dx.doi.org/10.7326/M22-1503",
"alternative-id": [
"10.7326/M22-1503"
],
"author": [
{
"affiliation": [],
"name": "ACTIV-3/TICO Study Group*",
"sequence": "first"
}
],
"container-title": "Annals of Internal Medicine",
"container-title-short": "Ann Intern Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
8,
8
]
],
"date-time": "2022-08-08T21:00:22Z",
"timestamp": 1659992422000
},
"deposited": {
"date-parts": [
[
2022,
8,
8
]
],
"date-time": "2022-08-08T21:00:25Z",
"timestamp": 1659992425000
},
"indexed": {
"date-parts": [
[
2022,
8,
8
]
],
"date-time": "2022-08-08T21:41:39Z",
"timestamp": 1659994899477
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
8,
9
]
]
},
"language": "en",
"member": "4285",
"original-title": [],
"prefix": "10.7326",
"published": {
"date-parts": [
[
2022,
8,
9
]
]
},
"published-online": {
"date-parts": [
[
2022,
8,
9
]
]
},
"publisher": "American College of Physicians",
"reference": [
{
"DOI": "10.1056/NEJMoa2107934",
"doi-asserted-by": "publisher",
"key": "r1-M221503"
},
{
"DOI": "10.1001/jama.2021.8828",
"doi-asserted-by": "publisher",
"key": "r2-M221503"
},
{
"DOI": "10.1056/NEJMoa2108163",
"doi-asserted-by": "publisher",
"key": "r3-M221503"
},
{
"DOI": "10.1056/NEJMoa2035002",
"doi-asserted-by": "publisher",
"key": "r4-M221503"
},
{
"DOI": "10.1056/NEJMoa2029849",
"doi-asserted-by": "publisher",
"key": "r5-M221503"
},
{
"DOI": "10.1016/S1473-3099(21)00751-9",
"doi-asserted-by": "publisher",
"key": "r6-M221503"
},
{
"DOI": "10.1056/NEJMoa2033130",
"doi-asserted-by": "publisher",
"key": "r7-M221503"
},
{
"DOI": "10.1016/S2213-2600(22)00215-6",
"doi-asserted-by": "publisher",
"key": "r8-M221503"
},
{
"DOI": "10.1056/NEJMc2119407",
"doi-asserted-by": "publisher",
"key": "r9-M221503"
},
{
"DOI": "10.1016/S0140-6736(22)00163-5",
"doi-asserted-by": "publisher",
"key": "r10-M221503"
},
{
"DOI": "10.1007/s40259-020-00429-8",
"doi-asserted-by": "publisher",
"key": "r11-M221503"
},
{
"DOI": "10.1038/nbt962",
"doi-asserted-by": "publisher",
"key": "r12-M221503"
},
{
"DOI": "10.1101/2021.02.03.429164",
"doi-asserted-by": "crossref",
"key": "r13-M221503",
"unstructured": "Rothenberger S, Hurdiss DL, Walser M, et al. Ensovibep, a novel trispecific DARPin candidate that protects against SARS-CoV-2 variants. bioRxiv. Preprint posted online 26 February 2022. doi:10.1101/2021.02.03.429164"
},
{
"DOI": "10.1101/2020.08.25.256339",
"doi-asserted-by": "crossref",
"key": "r14-M221503",
"unstructured": "Walser M, Rothenberger S, Hurdiss DL, et al. Highly potent anti-SARS-CoV-2 multivalent DARPin therapeutic candidates. bioRxiv. Preprint posted online 21 July 2021. doi:10.1101/2020.08.25.256339"
},
{
"DOI": "10.1177/17407745211049829",
"doi-asserted-by": "publisher",
"key": "r16-M221503"
},
{
"DOI": "10.1016/S2213-2600(19)30253-X",
"doi-asserted-by": "publisher",
"key": "r17-M221503"
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"doi-asserted-by": "publisher",
"key": "r18-M221503"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "r19-M221503"
},
{
"DOI": "10.1056/NEJMoa2102685",
"doi-asserted-by": "publisher",
"key": "r20-M221503"
},
{
"DOI": "10.1001/jama.2021.24939",
"doi-asserted-by": "publisher",
"key": "r21-M221503"
},
{
"DOI": "10.1016/S2213-2600(22)00180-1",
"doi-asserted-by": "publisher",
"key": "r22-M221503"
},
{
"DOI": "10.7326/M21-3507",
"doi-asserted-by": "publisher",
"key": "r23-M221503"
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.acpjournals.org/doi/10.7326/M22-1503"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine",
"Internal Medicine"
],
"subtitle": [
"A Randomized Controlled Trial"
],
"title": "Efficacy and Safety of Ensovibep for Adults Hospitalized With COVID-19",
"type": "journal-article"
}