N-acetylcysteine reduces severity and mortality in COVID-19 patients: A systematic review and meta-analysis
et al., Journal of Advanced Veterinary and Animal Research, doi:10.5455/javar.2023.j665, Jun 2023
16th treatment shown to reduce risk in
February 2021, now with p = 0.0000032 from 25 studies, recognized in 3 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
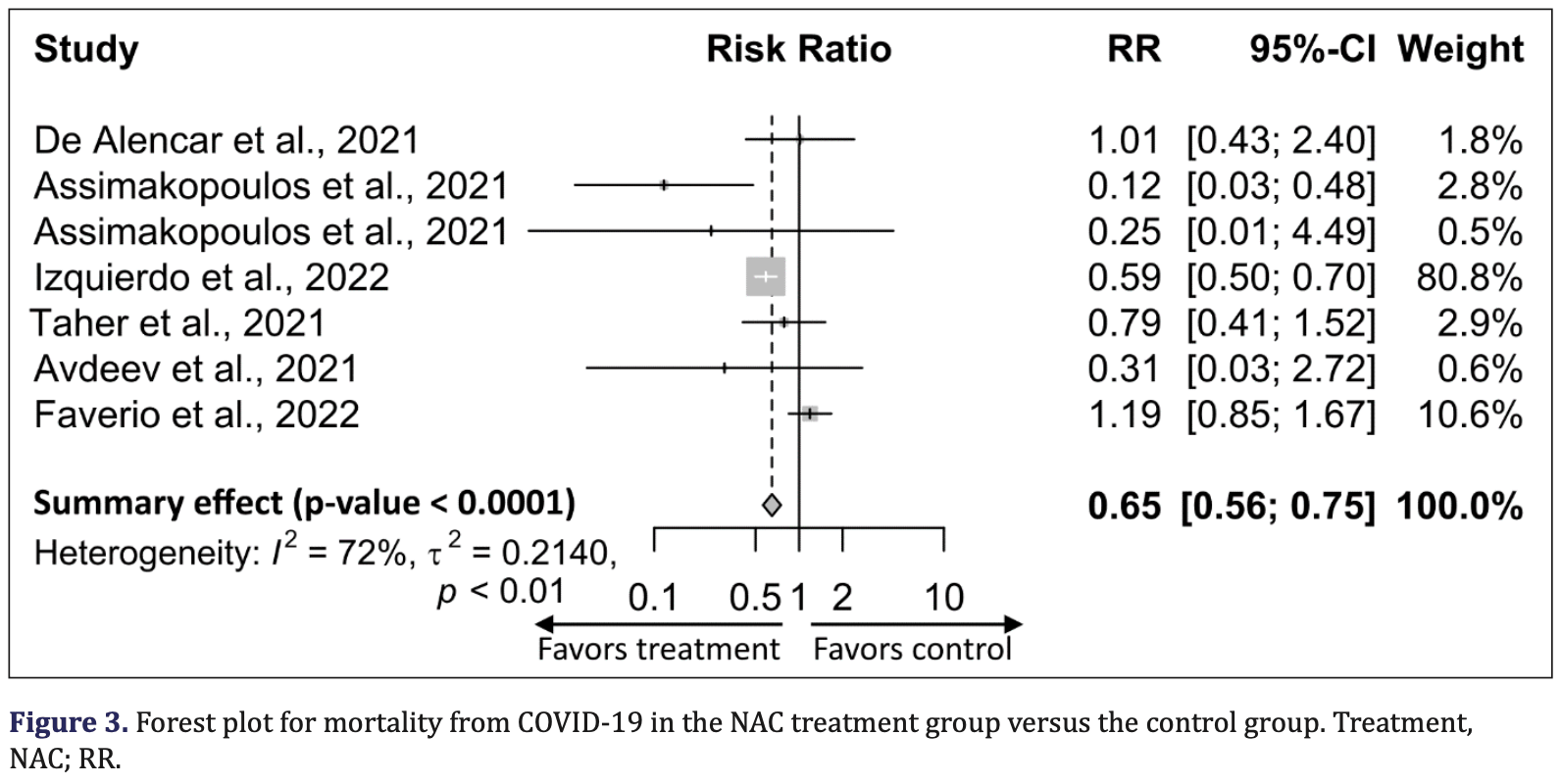
Systematic review and meta analysis showing lower mortality with N-acetylcysteine treatment.
Currently there are 25 N-acetylcysteine for COVID-19 studies, showing 31% lower mortality [14‑44%], 6% lower ventilation [-25‑29%], 7% lower ICU admission [-16‑26%], 11% lower hospitalization [6‑17%], and 28% fewer cases [21‑34%].
|
risk of death, 35.0% lower, RR 0.65, p < 0.001.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Alam et al., 30 Jun 2023, peer-reviewed, 6 authors.
Contact: shahalam@bsmrau.edu.bd, nazmol.stat.biotin@bsmrau.edu.bd.
N-acetylcysteine reduces severity and mortality in COVID-19 patients: A systematic review and meta-analysis
Journal of Advanced Veterinary and Animal Research, doi:10.5455/javar.2023.j665
Objectives: Recent clinical studies suggest that oxidative stress is one of the key players in the pathogenesis of coronavirus disease 2019 (COVID-19), and N-acetylcysteine (NAC), a potent antioxidant, has been shown to improve clinical outcomes in COVID-19 patients. We conducted a systematic review and meta-analysis of the literature published on the therapeutic intervention of NAC on COVID-19 infection. Methods: We searched PubMed, Google Scholar, and Science Direct. We identified and screened eight studies with 20,503 participants, including 2,852 in the NAC-treated group and 17,651 in the placebo group, which reported the effect of NAC on COVID-19 infection. A meta-analysis was performed using forest plots under fixed effect estimates based on the standardized mean difference (SMD) and risk ratio (RR). Results: Pooled analysis showed that NAC was associated with lower mortality in patients with COVID-19 compared with the placebo group [RR, 0.65; (95% CI: 0.56 to 0.75); p < 0.0001]. Similarly, C-reactive protein (CRP) [SMD, -0.32; (95% CI: -56 to -0.09); p = 0.0070] and D-dimer [SMD, -0.35, (95% CI: -0.59 to -0.10; p = 0.0062] levels were significantly decreased, and the oxygenation marker, PaO 2 /FiO 2 ratio, was increased in the NAC-treated group compared with the placebo group [SMD, 0.76; (95% CI: 0.48 to 1.03); p < 0.0001].
Conclusion: Although the number of included studies was minimal, this meta-analysis suggests that NAC may have a positive effect on COVID-19 outcomes, specifically, a significant decrease in CRP and D-dimer levels and a significant increase in oxygen saturation, which decreased mortality. We have also presented a comprehensive review of the role and mechanisms of NAC in patients with COVID-19.
Conflict of interests The authors declare that there is no conflict of interest in this paper.
Authors' contributions Mohammad Shah Alam was involved in the conception, data collection, drafting, intellectual reviewing, and overall article supervision. Mohammad Nazmol Hasan was involved in data collection, curation, and analysis. Mohammad Zahangeer Alam, KHM Nazmul Hussain Nazir, Fahima Khatun, and Zannatul Maowa assisted in editing the article.
References
Alam, Alam, Nazir, Bhuiyan, The emergence of novel coronavirus disease (COVID-19) in Bangladesh: present status, challenges, and future management, J Adv Vet Anim Res, doi:10.5455/javar.2020.g410
Alam, Czajkowsky, Islam, Rahman, The role of vitamin D in reducing SARS-CoV-2 infection: an update, Int Immunopharmacol, doi:10.1016/j.intimp.2021.107686
Alam, Czajkowsky, SARS-CoV-2 infection and oxidative stress: pathophysiological insight into thrombosis and therapeutic opportunities, Cytokine Growth Factor Rev, doi:1010.1016/j.cytogfr.2021.11.001
Alam, Insight into SARS-CoV-2 Omicron variant immune escape possibility and variant independent potential therapeutic opportunities, Heliyon, doi:10.1016/j.heliyon.2023.e13285
Alam, None, J. Adv. Vet. Anim. Res
Aldini, Altomare, Baron, Vistoli, Carini et al., N-acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why, Free Rad Res, doi:10.1080/10715762.2018.1468564
Andreou, Trantza, Filippou, Sipsas, Tsiodras, COVID-19: the potential role of copper and N-acetylcysteine (NAC) in a combination of candidate antiviral treatments against SARS-CoV-2, doi:10.21873/invivo.11946
Assimakopoulos, Aretha, Komninos, Dimitropoulou, Lagadinou et al., N-acetyl-cysteine reduces the risk for mechanical ventilation and mortality in patients with COVID-19 pneumonia: a two-center retrospective cohort study, Infect Dis, doi:10.1080/23744235.2021.1945675
Avdeev, Gaynitdinova, Merzhoeva, Berikkhanov, N-acetylcysteine for the treatment of COVID-19 among hospitalized patients, J Infect, doi:10.1016/j.jinf.2021.07.003
Calzetta, Matera, Rogliani, Cazzola, Multifaceted activity of N-acetyl-l-cysteine in chronic obstructive pulmonary disease, Exp Rev Respir Med, doi:10.1080/17476348.2018.1495562
Casale, COVID-19: can this crisis be transformative for global health?, Global Public Health, doi:10.1080/17441692.2020.1811366
Cecchini, Cecchini, SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression, Med Hypoth, doi:10.1016/j.mehy.2020.110102
Chen, Raja, Pierre-Louis, Patel, Patel et al., Intravenous N-acetylcysteine in management of COVID-19: a case series, J Pharm Pract, doi:10.1177/08971900221080283
Chen, Reheman, Gushiken, Nolasco, Fu et al., N-acetylcysteine reduces the size and activity of von Willebrand factor in human plasma and mice, J Clin Investig, doi:10.1172/JCI41062
Chidambaram, Tun, Haque, Majella, Sivakumar et al., Factors associated with disease severity and mortality among patients with COVID-19: a systematic review and meta-analysis, PloS One, doi:10.1371/journal.pone.0241541
Clinicaltrials, Gov, Inflammatory regulation effect of NAC on COVID-19 treatment (INFECT-19)
Cummings, Baldwin, Abrams, Jacobson, Meyer et al., Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York city: a prospective cohort study, Lancet, doi:10.1016/S0140-6736(20)31189-2
Darif, Hammi, Kihel, Saik, Guessous et al., The pro-inflammatory cytokines in COVID-19 pathogenesis: what goes wrong?, Microb Pathog, doi:10.1016/j.micpath.2021.104799
De Alencar, Moreira, Müller, Chaves, Fukuhara et al., Double-blind, randomized, placebo-controlled trial with N-acetylcysteine for treatment of severe acute respiratory syndrome caused by Coronavirus Disease 2019 (COVID-19), Clin Infect Dis, doi:10.1093/cid/ciaa1443
De Lizarrondo, Gakuba, Herbig, Repessé, Ali et al., Potent thrombolytic effect of N-acetylcysteine on arterial thrombi, Circulation, doi:10.1161/CIRCULATIONAHA.117.027290
Delgado-Roche, Mesta, Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection, Arch Med Res, doi:10.1016/j.arcmed.2020.04.019
Egger, Smith, Schneider, Minder, Bias in meta-analysis detected by a simple, graphical test, Br Med J, doi:10.1136/bmj.315.7109.629
Emert, Shah, Zampella, COVID-19 and hypercoagulability in the outpatient setting, Thromb Res, doi:10.1016/j.thromres.2020.05.031
Erel, Neşelioğlu, Tunçay, Ef, Eren et al., A sensitive indicator for the severity of COVID-19: thiol, Turk J Med Sci, doi:10.3906/sag-2011-139
Faverio, Rebora, Rossi, Giudice, Montanelli et al., Impact of N-acetyl-l-cysteine on SARS-CoV-2 pneumonia and its sequelae: results from a large cohort study, ERJ Open Res, doi:10.1183/23120541.00542-2021
Flora, Balansky, Maestra, Rationale for the use of N-acetylcysteine in both prevention and adjuvant therapy of
Flora, Grassi, Carati, Attenuation of influenza-like symptomatology and improvement of cell-mediated immunity with long-term N-acetylcysteine treatment, Eur Respir J, doi:10.1183/09031936.97.10071535
Frades, Miguel, Prieto, Ormaechea, Blas, The role of intermediate respiratory care units in preventing ICU collapse during the COVID pandemic, Int J Respir Pulm Med, doi:10.23937/2378-3516/1410147
Gaynitdinova, Avdeev, Merzhoeva, Berikkhanov, Medvedeva et al., N-acetylcysteine as a part of complex treatment of moderate COVID-associated pneumonia, Pulmonologiya, doi:10.18093/0869-0189-2021-31-1-21-29
Geiler, Michaelis, Naczk, Leutz, Langer et al., N-acetyl-L-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with Alam et al, doi:10.1016/j.bcp.2009.08.025
Griendling, Minieri, Ollerenshaw, Alexander, Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells, Circul Res, doi:10.1161/01.res.74.6.1141
Gupta, Madhavan, Sehgal, Nair, Mahajan et al., Extrapulmonary manifestations of COVID-19, Nat Med
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Horowitz, Freeman, Bruzzese, Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: a report of 2 cases, Respir Med Case Rep, doi:10.1016/j.rmcr.2020.101063
Hosakote, Liu, Castro, Garofalo, Casola, Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes, Am J Respir Cell Mol Biol, doi:10.1165/rcmb.2008-0330OC
Ibrahim, Smith, Lewis, Kon, Goldenberg, Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine, Clin Immunol, doi:10.1016/j.clim.2020.108544
Izquierdo, Soriano, González, Lumbreras, Ancochea et al., Use of N-acetylcysteine at high doses as an oral treatment for patients hospitalized with
Izquierdo-Alonso, Pérez-Rial, Rivera, Peces-Barba, N-acetylcysteine for prevention and treatment of COVID-19: current state of evidence and future directions, J Infect Public Health, doi:10.1016/j.jiph.2022.11.009
Jang, Weaver, Pizon, In vitro study of N-acetylcysteine on coagulation factors in plasma samples from healthy subjects, J Med Toxicol, doi:10.1007/s13181-012-0242-2
Khan, Campbell, Lu, An, Alpert et al., N-acetylcysteine for cardiac protection during coronary artery reperfusion: a systematic review and meta-analysis of randomized controlled trials, Front Cardiovasc Med, doi:10.3389/fcvm.2021.752939
Khomich, Kochetkov, Bartosch, Ivanov, Redox biology of respiratory viral infections, Viruses, doi:10.3390/v10080392
Labarrere, Kassab, Glutathione deficiency in the pathogenesis of SARS-CoV-2 infection and its effects upon the host immune response in severe COVID-19 disease, Front Microbiol, doi:10.3389/fmicb.2022.979719
Laforge, Elbim, Frère, Hémadi, Massaad et al., Tissue damage from neutrophil-induced oxidative stress in COVID-19, Nat Rev Immunol, doi:10.1038/s41577-020-0407-1
Mahase, Coronavirus, COVID-19 has killed more people than SARS and MERS combined, despite lower case fatality rate, Br Med J, doi:10.1136/bmj.m641
Mata, Morcillo, Gimeno, Cortijo, N-acetyl-L-cysteine (NAC) inhibit mucin synthesis and pro-inflammatory mediators in alveolar type II epithelial cells infected with influenza virus A and B and with respiratory syncytial virus (RSV), Biochem Pharmacol, doi:10.1016/j.bcp.2011.05.014
Meisinger, Kirchberger, Warm, Hyhlik-Dürr, Goßlau et al., Elevated plasma D-dimer concentrations in adults after an outpatient-treated COVID-19 infection, Viruses, doi:10.3390/v14112441
Moradi, Mojtahedzadeh, Mandegari, Soltan-Sharifi, Najafi et al., The role of glutathione-S-transferase polymorphisms on clinical outcome of ALI/ARDS patient treated with N-acetylcysteine, Respir Med, doi:10.1016/j.rmed.2008.09.013
Moreno-Solís, Aguilar, Torres-Borrego, Fj, Fernández-Gutiérrez et al., Oxidative stress and inflamatory plasma biomarkers in respiratory syncytial virus bronchiolitis, Clin Respir J, doi:10.1111/crj.12425
Mp, COVID-19 infection and oxidative stress: an under-explored approach for prevention and treatment?, Pan Afr Med J, doi:10.11604/pamj.2020.35.2.22877
Mp, N-acetylcysteine as a potential treatment for COVID-19, Future Microbiol, doi:10.2217/fmb-2020-0074
Nascimento, Suliman, Silva, Chinaglia, Marchioro et al., Effect of oral N-acetylcysteine treatment on plasma inflammatory and oxidative stress markers in peritoneal dialysis patients: a placebo-controlled study, Periton Dialys Int, doi:10.3747/pdi.2009.00073
Niemi, Munsterhjelm, Pöyhiä, Hynninen, Salmenperä, The effect of N-acetylcysteine on blood coagulation and platelet function in patients undergoing open repair of abdominal aortic aneurysm, Blood Coagul Fibrinol, doi:10.1097/01.mbc.0000195922.26950.89
Page, Mckenzie, Bossuyt, Boutron, Hoffmann et al., The PRISMA 2020 statement: an updated guideline for reporting systematic reviews, System Rev
Paraskevas, Kantanis, Karalis, Michailides, Karamouzos et al., N-acetylcysteine efficacy in patients hospitalized with COVID-19 pneumonia: a systematic review and meta-analysis, Romanian J Intern Med, doi:10.2478/rjim-2023-0001
Patel, Zhong, Grant, Oudit, Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure, Circul Res, doi:10.1161/CIRCRESAHA.116.307708
Peterson, Welch, Losos, Tugwell, The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, Ottawa, Ottawa Hosp Res Instit
Poe, Corn, N-acetylcysteine: a potential therapeutic agent for SARS-CoV-2, Med Hypoth, doi:10.1016/j.mehy.2020.109862
Polonikov, Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in COVID-19 patients, ACS Infect Dis, doi:10.1021/acsinfecdis.0c00288
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area, J Am Med Assoc, doi:10.1001/jama.2020.6775
Ruan, Yang, Wang, Jiang, Song, Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China, Intens Care Med, doi:10.1007/s00134-020-05991-x
Schwarzer, Meta-analysis in R, doi:10.1002/9781119099369.ch26
Sharafkhah, Abdolrazaghnejad, Zarinfar, Mohammadbeigi, Massoudifar et al., Safety and efficacy of N-acetyl-cysteine for prophylaxis of ventilator-associated pneumonia: a randomized, double blind, placebo-controlled clinical trial, Med Gas Res, doi:10.4103/2045-9912.229599
Shi, Puyo, N-acetylcysteine to combat COVID-19: an evidence review, Ther Clin Risk Manag, doi:10.2147/TCRM.S273700
Taher, Lashgari, Sedighi, Rahimi-Bashar, Poorolajal et al., A pilot study on intravenous N-acetylcysteine treatment in patients with mild-to-moderate COVID19-associated acute respiratory distress syndrome, Pharmacol Rep, doi:10.1007/s43440-021-00296-2
Tang, Li, Wang, Sun, Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia, J Thromb Haemost, doi:10.1111/jth.14768
Ullian, Gelasco, Fitzgibbon, Beck, Morinelli, N-acetylcysteine decreases angiotensin II receptor binding in vascular smooth muscle cells, J Am Soc Nephrol, doi:10.1681/ASN.2004060458
Wang, Aw, Stokes, N-acetylcysteine attenuates systemic platelet activation and cerebral vessel thrombosis in diabetes, Redox Biol, doi:10.1016/j.redox.2017.09.005
Wang, Sheng, Tu, Zhang, Association between peripheral lymphocyte count and the mortality risk of COVID-19 inpatients, BMC Pulm Med, doi:10.1186/s12890-021-01422-9
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese center for disease control and prevention, J Am Med Assoc, doi:10.1001/jama.2020.2648
Zaboli, Majidi, Alizadeh-Navaei, Hedayatizadeh-Omran, Asgarian-Omran et al., Lymphopenia and lung complications in patients with coronavirus disease-2019 (COVID-19): a retrospective study based on clinical data, J Med Virol, doi:10.1002/jmv.27060
Zhang, Davies, Forman, Oxidative stress response and Nrf2 signaling in aging, Free Rad Biol Med, doi:10.1016/j.freeradbiomed.2015.05.036
Zhang, Ding, Li, Wang, Chen et al., Effects of N-acetylcysteine treatment in acute respiratory distress syndrome: a meta-analysis, Exp Ther Med, doi:10.3892/etm.2017.4891
Zhang, Ju, Ma, Wang, N-acetylcysteine improves oxidative stress and inflammatory response in patients with community acquired pneumonia: a randomized controlled trial, Medicine, doi:10.1097/MD.0000000000013087
Çakırca, Çakırca, Üstünel, Torun, Koyuncu, Thiol level and total oxidant/antioxidant status in patients with COVID-19 infection, Irish J Med Sci, doi:10.1007/s11845-021-02743-8
DOI record:
{
"DOI": "10.5455/javar.2023.j665",
"ISSN": [
"2311-7710"
],
"URL": "http://dx.doi.org/10.5455/javar.2023.j665",
"abstract": "<jats:p>Objectives: Recent clinical studies suggest that oxidative stress is one of the key players in the pathogenesis of coronavirus disease 2019 (COVID-19), and N-acetylcysteine (NAC), a potent anti¬oxidant, has been shown to improve clinical outcomes in COVID-19 patients. We conducted a systematic review and meta-analysis of the literature published on the therapeutic intervention of NAC on COVID-19 infection. \nMethods: We searched PubMed, Google Scholar, and Science Direct. We identified and screened eight studies with 20,503 participants, including 2,852 in the NAC-treated group and 17,651 in the placebo group, which reported the effect of NAC on COVID-19 infection. A meta-analysis was performed using forest plots under fixed effect estimates based on the standardized mean difference (SMD) and risk ratio (RR). \nResults: Pooled analysis showed that NAC was associated with lower mortality in patients with COVID-19 compared with the placebo group [RR, 0.65; (95% CI: 0.56 to 0.75); p < 0.0001]. Similarly, C-reactive protein (CRP) [SMD, −0.32; (95% CI: −56 to −0.09); p = 0.0070] and D-dimer [SMD, −0.35, (95% CI: −0.59 to −0.10; p = 0.0062] levels were significantly decreased, and the oxygenation marker, PaO2/FiO2 ratio, was increased in the NAC-treated group compared with the placebo group [SMD, 0.76; (95% CI: 0.48 to 1.03); p < 0.0001]. \nConclusion: Although the number of included studies was minimal, this meta-analysis suggests that NAC may have a positive effect on COVID-19 outcomes, specifically, a significant decrease in CRP and D-dimer levels and a significant increase in oxygen saturation, which decreased mortality. We have also presented a comprehensive review of the role and mechanisms of NAC in patients with COVID-19.</jats:p>",
"author": [
{
"affiliation": [],
"family": "Alam",
"given": "Mohammad",
"sequence": "first"
},
{
"affiliation": [],
"family": "Hasan",
"given": "Mohammad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maowa",
"given": "Zannatul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khatun",
"given": "Fahima",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nazir",
"given": "K",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alam",
"given": "Mohammad",
"sequence": "additional"
}
],
"container-title": "Journal of Advanced Veterinary and Animal Research",
"container-title-short": "J Adv Vet Anim Res",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
7,
22
]
],
"date-time": "2023-07-22T17:52:57Z",
"timestamp": 1690048377000
},
"deposited": {
"date-parts": [
[
2023,
7,
22
]
],
"date-time": "2023-07-22T17:53:26Z",
"timestamp": 1690048406000
},
"indexed": {
"date-parts": [
[
2023,
7,
23
]
],
"date-time": "2023-07-23T04:24:49Z",
"timestamp": 1690086289165
},
"is-referenced-by-count": 0,
"issue": "2",
"issued": {
"date-parts": [
[
2023
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2023
]
]
}
},
"license": [
{
"URL": "https://bdvets.org/JAVAR/?sec=licenseinfo",
"content-version": "vor",
"delay-in-days": 202,
"start": {
"date-parts": [
[
2023,
7,
22
]
],
"date-time": "2023-07-22T00:00:00Z",
"timestamp": 1689984000000
}
}
],
"link": [
{
"URL": "https://www.ejmanager.com/fulltextpdf.php?mno=149309",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "3407",
"original-title": [],
"page": "157",
"prefix": "10.5455",
"published": {
"date-parts": [
[
2023
]
]
},
"published-print": {
"date-parts": [
[
2023
]
]
},
"publisher": "ScopeMed",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.ejmanager.com/fulltextpdf.php?mno=149309"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Veterinary",
"Animal Science and Zoology"
],
"subtitle": [],
"title": "N-acetylcysteine reduces severity and mortality in COVID-19 patients: A systematic review and meta-analysis",
"type": "journal-article",
"volume": "10"
}
