Effect of N-Acetylcysteine on mortality in COVID-19 patients: A systematic review and meta-analysis of randomized controlled trials
et al., Inflammopharmacology, doi:10.1007/s10787-025-01876-x, Jul 2025
16th treatment shown to reduce risk in
February 2021, now with p = 0.0000032 from 25 studies, recognized in 3 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
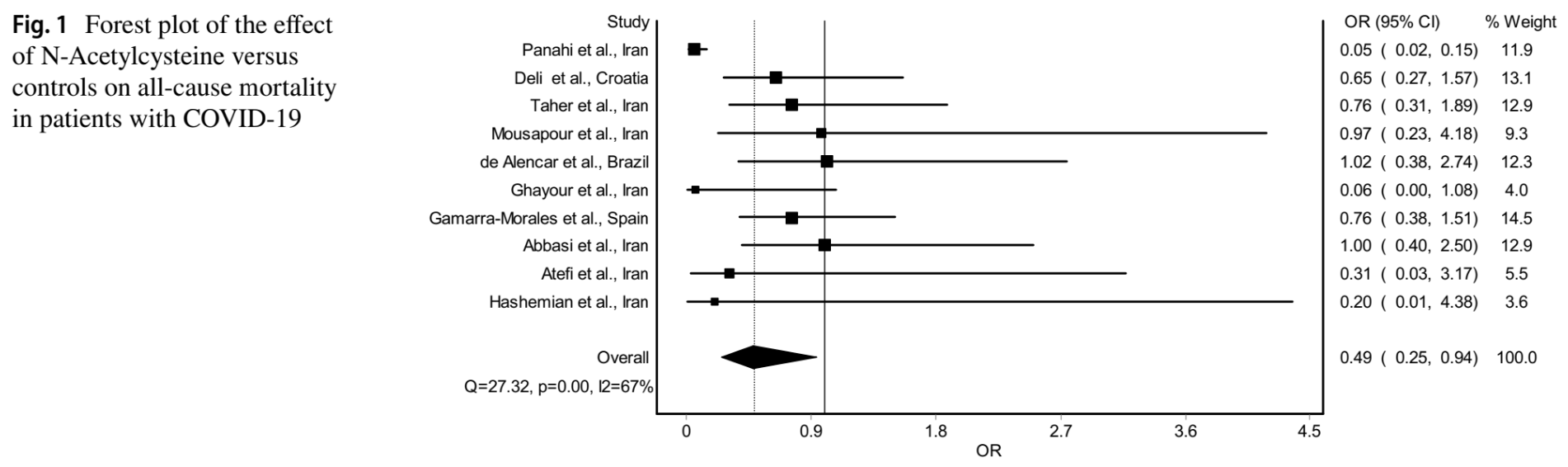
Meta analysis of 10 RCTs with 1,424 patients, showing significantly lower mortality with N-acetylcysteine.
Currently there are 25 N-acetylcysteine for COVID-19 studies, showing 31% lower mortality [14‑44%], 6% lower ventilation [-25‑29%], 7% lower ICU admission [-16‑26%], 11% lower hospitalization [6‑17%], and 28% fewer cases [21‑34%].
|
risk of death, 51.0% lower, OR 0.49, p = 0.03, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kow et al., 29 Jul 2025, peer-reviewed, 4 authors.
DOI record:
{
"DOI": "10.1007/s10787-025-01876-x",
"ISSN": [
"0925-4692",
"1568-5608"
],
"URL": "http://dx.doi.org/10.1007/s10787-025-01876-x",
"alternative-id": [
"1876"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "22 May 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "16 July 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "29 July 2025"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The authors have no relevant financial or non-financial interests to disclose."
}
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-8186-2926",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kow",
"given": "Chia Siang",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0001-5390-7026",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ramachandram",
"given": "Dinesh Sangarran",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-4058-2215",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hasan",
"given": "Syed Shahzad",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-3754-1690",
"affiliation": [],
"authenticated-orcid": false,
"family": "Thiruchelvam",
"given": "Kaeshaelya",
"sequence": "additional"
}
],
"container-title": "Inflammopharmacology",
"container-title-short": "Inflammopharmacol",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
7,
29
]
],
"date-time": "2025-07-29T08:11:34Z",
"timestamp": 1753776694000
},
"deposited": {
"date-parts": [
[
2025,
7,
30
]
],
"date-time": "2025-07-30T23:05:16Z",
"timestamp": 1753916716000
},
"indexed": {
"date-parts": [
[
2025,
7,
30
]
],
"date-time": "2025-07-30T23:40:03Z",
"timestamp": 1753918803751,
"version": "3.41.2"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
7,
29
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.springernature.com/gp/researchers/text-and-data-mining",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
7,
29
]
],
"date-time": "2025-07-29T00:00:00Z",
"timestamp": 1753747200000
}
},
{
"URL": "https://www.springernature.com/gp/researchers/text-and-data-mining",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
7,
29
]
],
"date-time": "2025-07-29T00:00:00Z",
"timestamp": 1753747200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s10787-025-01876-x.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s10787-025-01876-x/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s10787-025-01876-x.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1007",
"published": {
"date-parts": [
[
2025,
7,
29
]
]
},
"published-online": {
"date-parts": [
[
2025,
7,
29
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"author": "S Abbasi",
"first-page": "217",
"journal-title": "Int J Med Lab",
"key": "1876_CR1",
"unstructured": "Abbasi S, Fani M, Sayar S, Radmanesh E, Jelvay S, Pahlavanzadeh B et al (2023) The effects of vitamin D3 and N-acetylcysteine administration in patients with COVID-19 hospitalization in the Iranian population. Int J Med Lab 10:217–228",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1002/iid3.1083",
"author": "N Atefi",
"doi-asserted-by": "publisher",
"issue": "11",
"journal-title": "Immun Inflamm Dis",
"key": "1876_CR2",
"unstructured": "Atefi N, Goodarzi A, Riahi T et al (2023) Evaluation of the efficacy and safety of oral N-acetylcysteine in patients with COVID-19 receiving the routine antiviral and hydroxychloroquine protocol: a randomized controlled clinical trial. Immun Inflamm Dis 11(11):e1083. https://doi.org/10.1002/iid3.1083",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1016/j.coph.2007.04.005",
"author": "KR Atkuri",
"doi-asserted-by": "publisher",
"first-page": "355",
"issue": "4",
"journal-title": "Curr Opin Pharmacol",
"key": "1876_CR3",
"unstructured": "Atkuri KR, Mantovani JJ, Herzenberg LA, Herzenberg LA (2007) N-acetylcysteine—a safe antidote for cysteine/glutathione deficiency. Curr Opin Pharmacol 7(4):355–359. https://doi.org/10.1016/j.coph.2007.04.005",
"volume": "7",
"year": "2007"
},
{
"DOI": "10.1097/JCMA.0000000000000869",
"author": "CH Chen",
"doi-asserted-by": "crossref",
"first-page": "274",
"issue": "3",
"journal-title": "J Chin Med Assoc",
"key": "1876_CR4",
"unstructured": "Chen CH, Hung KF, Huang CY, Leong JL, Chu YC, Chang CY, Wang ML, Chiou SH, Cheng YF (2023) Is N-acetylcysteine effective in treating patients with coronavirus disease 2019? A meta-analysis. J Chin Med Assoc 86(3):274–281",
"volume": "86",
"year": "2023"
},
{
"key": "1876_CR5",
"unstructured": "Cochrane Methods Bias. (2023). RoB 2: A revised Cochrane risk-of-bias tool for randomized trials. https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials"
},
{
"DOI": "10.1093/cid/ciaa1443",
"author": "de Alencar, J. C. G., Moreira, C. L., Müller, A. D., Chaves, C. E., Fukuhara, M. A., da Silva, E. A., … COVID Register Group",
"doi-asserted-by": "crossref",
"first-page": "e736",
"issue": "11",
"journal-title": "Clin Infect Dis",
"key": "1876_CR6",
"unstructured": "de Alencar, J. C. G., Moreira, C. L., Müller, A. D., Chaves, C. E., Fukuhara, M. A., da Silva, E. A., … COVID Register Group (2021) Double-blind, randomized, placebo-controlled trial with N-acetylcysteine for treatment of severe acute respiratory syndrome caused by coronavirus disease 2019 (COVID-19). Clin Infect Dis 72(11):e736–e741",
"volume": "72",
"year": "2021"
},
{
"DOI": "10.3390/microorganisms10061118",
"author": "N Delić",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "Microorganisms",
"key": "1876_CR7",
"unstructured": "Delić N, Matetic A, Domjanović J, Kljaković-Gašpić T, Šarić L, Ilić D, Došenović S, Domazet J, Kovač R, Runjić F, Stipić SS, Duplančić B (2022) Effects of different inhalation therapy on ventilator-associated pneumonia in ventilated COVID-19 patients: a randomized controlled trial. Microorganisms 10(6):1118",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.3390/antiox11030445",
"author": "S Eligini",
"doi-asserted-by": "publisher",
"first-page": "445",
"issue": "3",
"journal-title": "Antioxidants",
"key": "1876_CR8",
"unstructured": "Eligini S, Porro B, Aldini G, Colli S, Banfi C (2022) N-acetylcysteine inhibits platelet function through the regeneration of the non-oxidative form of albumin. Antioxidants 11(3):445. https://doi.org/10.3390/antiox11030445",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.5501/wjv.v11.i1.82",
"author": "J Finsterer",
"doi-asserted-by": "publisher",
"first-page": "82",
"issue": "1",
"journal-title": "World J Virol",
"key": "1876_CR9",
"unstructured": "Finsterer J, Scorza FA, Scorza CA, Fiorini AC (2022) Repurposing the antioxidant and anti-inflammatory agent N-acetyl cysteine for treating COVID-19. World J Virol 11(1):82–84. https://doi.org/10.5501/wjv.v11.i1.82",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.3390/nu15092235",
"author": "Y Gamarra-Morales",
"doi-asserted-by": "crossref",
"issue": "9",
"journal-title": "Nutrients",
"key": "1876_CR10",
"unstructured": "Gamarra-Morales Y, Herrera-Quintana L, Molina-López J, Vázquez-Lorente H, Machado-Casas JF, Castaño-Pérez J, Pérez-Villares JM, Planells E (2023) Response to intravenous N-acetylcysteine supplementation in critically ill patients with COVID-19. Nutrients 15(9):2235",
"volume": "15",
"year": "2023"
},
{
"author": "AE Ghayour",
"first-page": "86",
"issue": "2",
"journal-title": "Revista Clínica Española (English Edition)",
"key": "1876_CR11",
"unstructured": "Ghayour AE, Nazari S, Keramat F, Shahbazi F, Eslami-Ghayour A (2024) Evaluation of the efficacy of N-acetylcysteine and bromhexine compared with standard care in preventing hospitalization of outpatients with COVID-19: a double blind randomized clinical trial. Revista Clínica Española (English Edition) 224(2):86–95",
"volume": "224",
"year": "2024"
},
{
"DOI": "10.5694/mja2.50674",
"author": "PG Gibson",
"doi-asserted-by": "publisher",
"first-page": "54",
"issue": "2",
"journal-title": "Med J Aust",
"key": "1876_CR12",
"unstructured": "Gibson PG, Qin L, Puah SH (2020) COVID-19 acute respiratory distress syndrome (ARDS): Clinical features and differences from typical pre-COVID-19 ARDS. Med J Aust 213(2):54-56.e1. https://doi.org/10.5694/mja2.50674",
"volume": "213",
"year": "2020"
},
{
"author": "H Hashemian",
"issue": "2",
"journal-title": "J Comprehen Pediatr",
"key": "1876_CR13",
"unstructured": "Hashemian H, Qobadighadikolaei R, Seifnezhad P, Rad AH, Mansouri SS, Darini A, Shahrokhi M (2024) Efficacy of N-acetylcysteine in children with moderate COVID-19: A placebo-controlled randomized clinical trial. J Comprehen Pediatr 15(2):e139612",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1056/NEJMct0708278",
"author": "KJ Heard",
"doi-asserted-by": "publisher",
"first-page": "285",
"issue": "3",
"journal-title": "N Engl J Med",
"key": "1876_CR14",
"unstructured": "Heard KJ (2008) Acetylcysteine for acetaminophen poisoning. N Engl J Med 359(3):285–292. https://doi.org/10.1056/NEJMct0708278",
"volume": "359",
"year": "2008"
},
{
"DOI": "10.1016/j.jiph.2022.11.009",
"author": "JL Izquierdo-Alonso",
"doi-asserted-by": "publisher",
"first-page": "1477",
"issue": "12",
"journal-title": "J Infect Public Health",
"key": "1876_CR15",
"unstructured": "Izquierdo-Alonso JL, Pérez-Rial S, Rivera CG, Peces-Barba G (2022) N-acetylcysteine for prevention and treatment of COVID-19: current state of evidence and future directions. J Infect Public Health 15(12):1477–1483. https://doi.org/10.1016/j.jiph.2022.11.009",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1038/sj.npp.1301624",
"author": "S Lavoie",
"doi-asserted-by": "publisher",
"first-page": "2187",
"issue": "9",
"journal-title": "Neuropsychopharmacology",
"key": "1876_CR16",
"unstructured": "Lavoie S, Murray MM, Deppen P, Knyazeva MG, Berk M, Boulat O, Bovet P, Bush AI, Conus P, Copolov D, Fornari E, Meuli R, Solida A, Vianin P, Cuénod M, Buclin T, Do KQ (2008) Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology 33(9):2187–2199. https://doi.org/10.1038/sj.npp.1301624",
"volume": "33",
"year": "2008"
},
{
"author": "TH Liu",
"issue": "3",
"journal-title": "Heliyon",
"key": "1876_CR17",
"unstructured": "Liu TH, Wu JY, Huang PY, Tsai YW, Hsu WH, Chuang MH, Tang HJ, Lai CC (2024) Clinical efficacy of N-acetylcysteine for COVID-19: a systematic review and meta-analysis of randomized controlled trials. Heliyon 10(3):e25179",
"volume": "10",
"year": "2024"
},
{
"DOI": "10.3390/antiox12091713",
"author": "D Mokra",
"doi-asserted-by": "publisher",
"issue": "9",
"journal-title": "Antioxidants",
"key": "1876_CR18",
"unstructured": "Mokra D, Mokry J, Barosova R, Hanusrichterova J (2023) Advances in the use of N-acetylcysteine in chronic respiratory diseases. Antioxidants 12(9):1713. https://doi.org/10.3390/antiox12091713",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.3390/ijms26062657",
"author": "D Mokra",
"doi-asserted-by": "publisher",
"issue": "6",
"journal-title": "Int J Mol Sci",
"key": "1876_CR19",
"unstructured": "Mokra D, Porvaznik I, Mokry J (2025) N-acetylcysteine in the treatment of acute lung injury: perspectives and limitations. Int J Mol Sci 26(6):2657. https://doi.org/10.3390/ijms26062657",
"volume": "26",
"year": "2025"
},
{
"author": "P Mousapour",
"first-page": "241",
"issue": "3",
"journal-title": "Gastroenterol Hepatol Bed Bench",
"key": "1876_CR20",
"unstructured": "Mousapour P, Hamidi Farahani R, Mosaed R, Asgari A, Hazrati E (2022) Efficacy and safety of acetylcysteine for the prevention of liver injury in COVID-19 intensive care unit patients under treatment with remdesivir. Gastroenterol Hepatol Bed Bench 15(3):241–248",
"volume": "15",
"year": "2022"
},
{
"author": "MJ Page",
"journal-title": "BMJ",
"key": "1876_CR21",
"unstructured": "Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372:n71",
"volume": "372",
"year": "2021"
},
{
"DOI": "10.1002/jmv.28393",
"author": "Y Panahi",
"doi-asserted-by": "crossref",
"first-page": "e28393",
"issue": "1",
"journal-title": "J Med Virol",
"key": "1876_CR22",
"unstructured": "Panahi Y, Ghanei M, Rahimi M, Samim A, Vahedian-Azimi A, Atkin SL, Sahebkar A (2023) Evaluation of the efficacy and safety of N-acetylcysteine inhalation spray in controlling the symptoms of patients with COVID-19: an open-label randomized controlled clinical trial. J Med Virol 95(1):e28393",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.3390/jcm13144127",
"author": "P Santus",
"doi-asserted-by": "publisher",
"first-page": "4127",
"issue": "14",
"journal-title": "J Clin Med",
"key": "1876_CR23",
"unstructured": "Santus P, Signorello JC, Danzo F, Lazzaroni G, Saad M, Radovanovic D (2024) Anti-inflammatory and anti-oxidant properties of N-acetylcysteine: a fresh perspective. J Clin Med 13(14):4127. https://doi.org/10.3390/jcm13144127",
"volume": "13",
"year": "2024"
},
{
"DOI": "10.2147/TCRM.S273700",
"author": "Z Shi",
"doi-asserted-by": "publisher",
"first-page": "1047",
"journal-title": "Ther Clin Risk Manag",
"key": "1876_CR24",
"unstructured": "Shi Z, Puyo CA (2020) N-acetylcysteine to combat COVID-19: an evidence review. Ther Clin Risk Manag 16:1047–1055. https://doi.org/10.2147/TCRM.S273700",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.3390/v15020553",
"author": "MJA Silva",
"doi-asserted-by": "publisher",
"first-page": "553",
"issue": "2",
"journal-title": "Viruses",
"key": "1876_CR25",
"unstructured": "Silva MJA, Ribeiro LR, Gouveia MIM, Marcelino BDR, Santos CSD, Lima KVB, Lima LNGC (2023) Hyperinflammatory response in COVID-19: a systematic review. Viruses 15(2):553. https://doi.org/10.3390/v15020553",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1007/s43440-021-00296-2",
"author": "A Taher",
"doi-asserted-by": "crossref",
"first-page": "1650",
"issue": "6",
"journal-title": "Pharmacol Rep",
"key": "1876_CR26",
"unstructured": "Taher A, Lashgari M, Sedighi L, Rahimi-Bashar F, Poorolajal J, Mehrpooya M (2021) A pilot study on intravenous N-acetylcysteine treatment in patients with mild-to-moderate COVID-19-associated acute respiratory distress syndrome. Pharmacol Rep 73(6):1650–1659",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1016/j.cpcardiol.2020.100618",
"author": "S Zaim",
"doi-asserted-by": "publisher",
"issue": "8",
"journal-title": "Curr Probl Cardiol",
"key": "1876_CR27",
"unstructured": "Zaim S, Chong JH, Sankaranarayanan V, Harky A (2020) COVID-19 and multiorgan response. Curr Probl Cardiol 45(8):100618. https://doi.org/10.1016/j.cpcardiol.2020.100618",
"volume": "45",
"year": "2020"
}
],
"reference-count": 27,
"references-count": 27,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s10787-025-01876-x"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Effect of N-Acetylcysteine on mortality in COVID-19 patients: A systematic review and meta-analysis of randomized controlled trials",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy"
}
