Effectiveness of dolutegravir in moderate severity COVID-19 patients: A single-center, randomized, double-blind, placebo-controlled trial
et al., BioImpacts, doi:10.34172/bi.29952, IRCT20200328046886N3, Jun 2024
RCT 93 hospitalized patients with moderate COVID-19 showing no significant difference in clinical outcomes with dolutegravir treatment compared to placebo.
|
risk of death, 202.2% higher, RR 3.02, p = 0.49, treatment 1 of 46 (2.2%), control 0 of 47 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
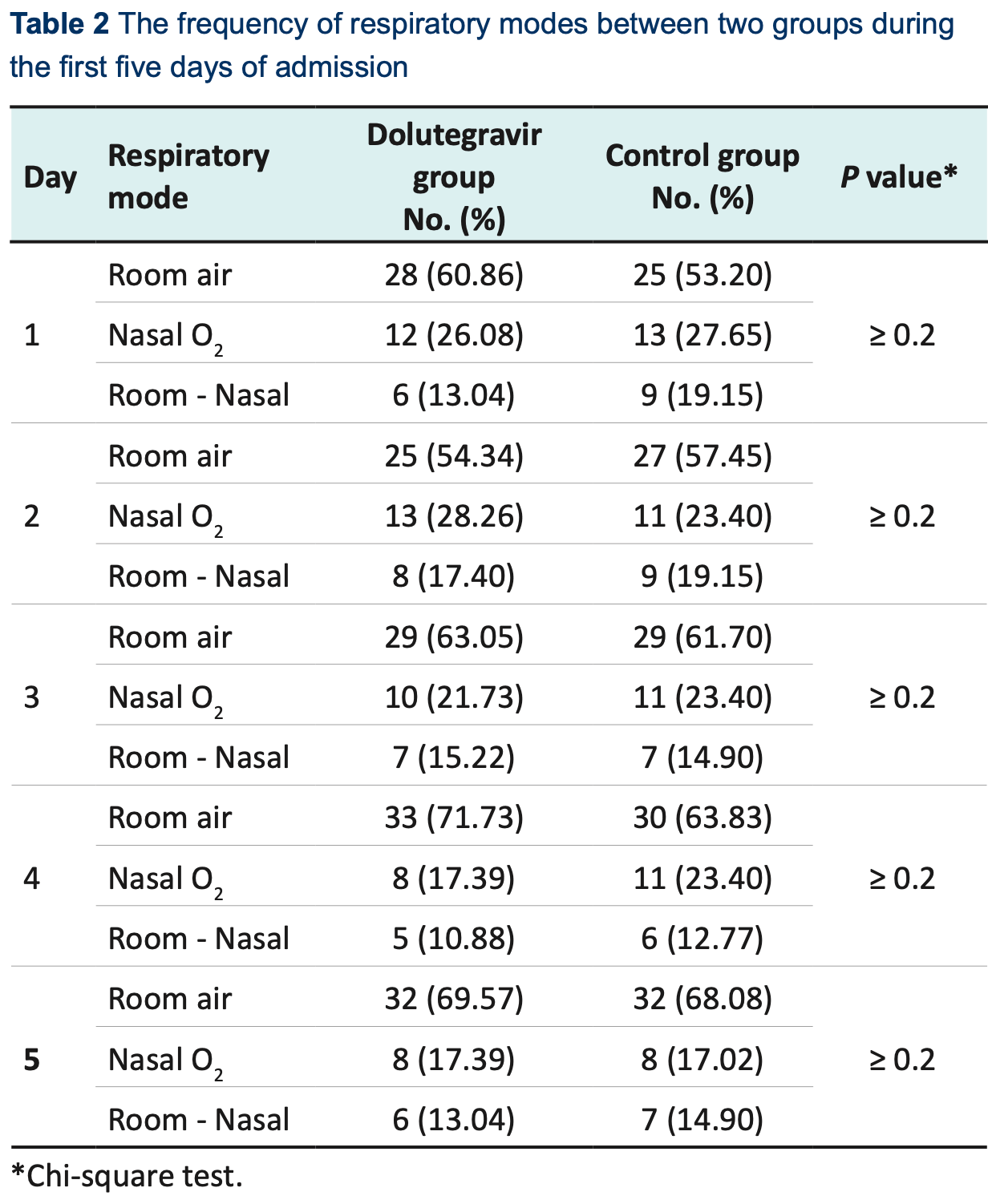
risk of oxygen therapy, 4.6% lower, RR 0.95, p = 1.00, treatment 14 of 46 (30.4%), control 15 of 47 (31.9%), NNT 68, day 5.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Abbaspour Kasgari et al., 26 Jun 2024, Double Blind Randomized Controlled Trial, placebo-controlled, Iran, peer-reviewed, mean age 49.0, 4 authors, study period 22 August, 2021 - 23 October, 2021, average treatment delay 7.0 days, trial IRCT20200328046886N3.
Contact: nasimahmadian@ut.ac.ir.
Effectiveness of dolutegravir in moderate severity COVID-19 patients: A single-center, randomized, double-blind, placebo-controlled trial
BioImpacts, doi:10.34172/bi.29952
Introduction: Drug repurposing as a low-cost, time-saving, and often less risky strategy has been attractive for the treatment of coronavirus disease 2019 (COVID-19) during the pandemic. This trial aimed to evaluate the effectiveness of dolutegravir, an HIV-1 integrase inhibitor, in admitted patients with moderate COVID-19. Methods: This study was a randomized, double-blind, placebo-controlled clinical trial assessing the efficacy of dolutegravir in adults admitted to a hospital in Ghaemshahr, Mazandaran Province, Iran. Patients aged 18-80 years with early symptoms of moderate COVID-19, which was confirmed based on reverse transcription polymerase chain reaction (RT-PCR) and/or chest computed tomography (CT) scan, were considered to be included in this study. Patients were randomly assigned in a 1:1 ratio to receive 50 mg dolutegravir plus the standard treatment regimen or the same value of placebo plus the standard treatment regimen, daily for 7 days. The standard treatment regimen was remdesivir 200 mg on day 1 followed by 100 mg for five days or until discharge. The primary endpoint was recovery 10 days after the beginning of the study. Results: Between August 22 and October 23, 2021, of 120 patients who were enrolled, 93 patients were randomly assigned to receive 50 mg dolutegravir (n = 46) or the placebo regimen (n = 47). No significant difference was observed between the two intervention groups based on the obtained results including frequency of respiratory modes during the first five days of admission, respiratory rate, and O 2 saturation during six time periods.
Conclusion: The results showed that in adult patients admitted to the hospital with moderate COVID-19, treatment with dolutegravir was not associated with improvement in clinical recovery. Larger randomized trials are required to provide more robust evidence about the effectiveness of dolutegravir.
Competing Interests The authors declare that there is no conflict of interest.
Ethical Statement The
References
Aghaali, Kolifarhood, Nikbakht, Saadati, Nazari, Estimation of the serial interval and basic reproduction number of COVID-19 in Qom, Iran, and three other countries: A data-driven analysis in the early phase of the outbreak, Transbound Emerg Dis, doi:10.1111/tbed.13656
Ali, Hanif, Haider, Ahmed, Sundas et al., Treatment options for COVID-19: a review, Front Med, doi:10.3389/fmed.2020.00480
Amin, Banerjee, Ghosh, Gayen, Jha, Protease targeted COVID-19 drug discovery and its challenges: Insight into viral main protease (Mpro) and papain-like protease (PLpro) inhibitors, Bioorg Med Chem, doi:10.1016/j.bmc.2020.115860
Baig, Khaleeq, Ali, Syeda, Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms, ACS Chem Neurosci, doi:10.1021/acschemneuro.0c00122
Bakowski, Beutler, Wolff, Kirkpatrick, Chen et al., Drug repurposing screens identify chemical entities for the development of COVID-19 interventions, Nat Commun, doi:10.1038/s41467-021-23328-0
Barouch, Covid-19 vaccines-immunity, variants, boosters, N Engl J Med, doi:10.1056/NEJMra2206573
Behera, Mahapatra, Tripathy, Pati, Drug repurposing for identification of potential inhibitors against SARS-CoV-2 spike receptor-binding domain: An in silico approach, J Biomol Struct Dyn, doi:10.4103/ijmr.IJMR_1132_20
Cahn, Candidates for inclusion in a universal antiretroviral regimen: dolutegravir, Curr Opin HIV AIDS, doi:10.1097/COH.0000000000000388
Chakraborty, Sharma, Bhattacharya, Lee, A detailed overview of immune escape, antibody escape, partial vaccine escape of SARS-CoV-2 and their emerging variants with escape mutations, Front Immunol, doi:10.3389/fimmu.2022.801522
Chen, Liu, Guo, Emerging coronaviruses: genome structure, replication, and pathogenesis, J Med Virol, doi:10.1002/jmv.25681
Chen, Xiong, Bao, Shi, Convalescent plasma as a potential therapy for COVID-19, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30141-9
Dastan, Saffaei, Haseli, Marjani, Moniri et al., Promising effects of tocilizumab in COVID-19: a noncontrolled, prospective clinical trial, Int Immunopharmacol, doi:10.1016/j.intimp.2020.106869
Delgado, Duro, Rogers, Tkatchenko, Pandit et al., Molecular basis for higher affinity of SARS-CoV-2 spike RBD for human ACE2 receptor, Proteins, doi:10.1002/prot.26086
Drożdżal, Rosik, Lechowicz, Machaj, Kotfis et al., FDA approved drugs with pharmacotherapeutic potential for SARS-CoV-2 (COVID-19) therapy, Drug Resist Updat, doi:10.1016/j.drup.2020.100719
Duan, Liu, Li, Zhang, Yu et al., Effectiveness of convalescent plasma therapy in severe COVID-19 patients, Proc Natl Acad Sci U S A, doi:10.1073/pnas.2004168117
Dubé, Ward, Verger, Macdonald, Vaccine hesitancy, acceptance, and anti-vaccination: trends and future prospects for public health, Annu Rev Public Health, doi:10.1146/annurev-publhealth-090419-102240
Fattahi, Mohseni, Jalalvand, Moghadam, Ghaziasadi et al., SARS-CoV-2 outbreak in Iran: The dynamics of the epidemic and evidence on two independent introductions, Transbound Emerg Dis, doi:10.1111/tbed.14104
Finsterer, Neurological side effects of SARS-CoV-2 vaccinations, Acta Neurol Scand, doi:10.1111/ane.13550
Galindez, Matschinske, Rose, Sadegh, Salgado-Albarrán et al., Lessons from the COVID-19 pandemic for advancing computational drug repurposing strategies, Nat Comput Sci, doi:10.1038/s43588-020-00007-6
Gao, Yan, Huang, Liu, Zhao et al., Structure of the RNA-dependent RNA polymerase from COVID-19 virus, Science, doi:10.1126/science.abb7498
Garrepalli, Gudipati, Kapavarapu, Ravindhranath, Pal, Synthesis and characterization of two known and one new impurities of dolutegravir: In silico evaluation of certain intermediates against SARS CoV-2 O-ribose methyltransferase (OMTase), Journal of Molecular Structure, doi:10.1016/j.molstruc.2022.133992
Gysi, Valle, Zitnik, Ameli, Gan et al., Network medicine framework for identifying drug-repurposing opportunities for COVID-19, Proc Natl Acad Sci U S A, doi:10.1073/pnas.2025581118
Hasanzad, Namazi, Larijani, COVID-19 anti-vaccine attitude and hesitancy, Journal of Diabetes & Metabolic Disorders, doi:10.1007/s40200-022-01018-y
Hogan, Pardi, mRNA Vaccines in the COVID-19 Pandemic and Beyond, Annu Rev Med, doi:10.1146/annurev-med-042420-112725
Hosseini, Askari, A review of neurological side effects of COVID-19 vaccination, Eur J Med Res, doi:10.1186/s40001-023-00992-0
Indu, Rameshkumar, Arunagirinathan, Al-Dhabi, Arasu et al., Raltegravir, Indinavir, Tipranavir, Dolutegravir, and Etravirine against main protease and RNAdependent RNA polymerase of SARS-CoV-2: A molecular docking and drug repurposing approach, J Infect Public Health, doi:10.1016/j.jiph.2020.10.015
Kalantari, Fard, Maleki, Taher, Yassin et al., Comparing the effectiveness of Atazanavir/Ritonavir/ Dolutegravir/Hydroxychloroquine and Lopinavir/Ritonavir/ Hydroxychloroquine treatment regimens in COVID-19 patients, J Med Virol, doi:10.1002/jmv.27195
Katlama, Murphy, Dolutegravir for the treatment of HIV, Expert Opin Investig Drugs, doi:10.1517/13543784.2012.661713
Lan, Ge, Yu, Shan, Zhou et al., Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor, Nature, doi:10.1038/s41586-020-2180-5
Lee, Chong, Sukumaran, Nimmanpipug, Letchumanan et al., Computational screening and identifying binding interaction of anti-viral and anti-malarial drugs: Toward the potential cure for SARS-CoV-2, Progress in Drug Discovery & Biomedical Science, doi:10.36877/pddbs.a0000065
Li, Clercq, Therapeutic options for the 2019 novel coronavirus (2019-nCoV), Nat Rev Drug Discov, doi:10.1038/d41573-020-00016-0
Llibre, Hung, Brinson, Castelli, Girard et al., Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies, Lancet, doi:10.1016/S0140-6736(17)33095-7
Lu, Zhao, Li, Niu, Yang et al., Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding, Lancet, doi:10.1016/S0140-6736(20)30251-8
Luo, Wu, Yu, Chen, Zhou, Novel coronavirus mutations: Vaccine development and challenges, Microb Pathog, doi:10.1016/j.micpath.2022.105828
Macchiagodena, Pagliai, Procacci, Identification of potential binders of the main protease 3CLpro of the COVID-19 via structure-based ligand design and molecular modeling, Chemical Physics Letters, doi:10.1016/j.cplett.2020.137489
Menachery, Schäfer, Burnum-Johnson, Mitchell, Eisfeld et al., MERS-CoV and H5N1 influenza virus antagonize antigen presentation by altering the epigenetic landscape, Proc Natl Acad Sci U S A, doi:10.1073/pnas.1706928115
Mohamed, Yazdanpanah, Saghazadeh, Rezaei, Computational drug discovery and repurposing for the treatment of COVID-19: a systematic review, Bioorg Chem, doi:10.1016/j.bioorg.2020.104490
Murugan, Kumar, Jeyakanthan, Srivastava, Searching for target-specific and multi-targeting organics for Covid-19 in the Drugbank database with a double scoring approach, Sci Rep, doi:10.1038/s41598-020-75762-7
Oprea, Bauman, Bologa, Buranda, Chigaev et al., Drug repurposing from an academic perspective, Drug Discovery Today: Therapeutic Strategies, doi:10.1016/j.ddstr.2011.10.002
Osterholzer, Goldman, Dolutegravir: a next-generation integrase inhibitor for treatment of HIV infection, Clin Infect Dis, doi:10.1093/cid/ciu221
Poustchi, Darvishian, Mohammadi, Shayanrad, Delavari et al., SARS-CoV-2 antibody seroprevalence in the general population and high-risk occupational groups across 18 cities in Iran: a population-based cross-sectional study, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30858-6
Pushpakom, Iorio, Eyers, Escott, Hopper et al., Drug repurposing: progress, challenges and recommendations, Nat Rev Drug Discov, doi:10.1038/nrd.2018.168
Rathbun, Lockhart, Miller, Liedtke, Dolutegravir, a second-generation integrase inhibitor for the treatment of HIV-1 infection, Ann Pharmacother, doi:10.1177/1060028013513558
Rhee, Grant, Tzou, Barrow, Harrigan et al., A systematic review of the genetic mechanisms of dolutegravir resistance, J Antimicrob Chemother, doi:10.1093/jac/dkz256
Rothan, Byrareddy, The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak, J Autoimmun, doi:10.1016/j.jaut.2020.102433
Rudrapal, Khairnar, Jadhav, Drug repurposing (DR): an emerging approach in drug discovery. drug repurposinghypothesis, molecular aspects and therapeutic applications IntechOpen, Intech Open Publications, doi:10.5772/intechopen.93193
Sahraei, Shabani, Shokouhi, Saffaei, Aminoquinolines against coronavirus disease 2019 (COVID-19): chloroquine or hydroxychloroquine, Int J Antimicrob Agents
Senanayake, Drug repurposing strategies for COVID-19, Future Science, doi:10.4155/fdd-2020-0010
Shang, Ye, Shi, Wan, Luo et al., Structural basis of receptor recognition by SARS-CoV-2, Nature, doi:10.1038/s41586-020-2179-y
Sharma, Deep, In-silico drug repurposing for targeting SARS-CoV-2 main protease (Mpro), J Biomol Struct Dyn, doi:10.1080/07391102.2020.1844058
Simmons, Wentzel, Mobarak, Eslami, Sadeghi et al., Sofosbuvir/daclatasvir regimens for the treatment of COVID-19: an individual patient data meta-analysis, J Antimicrob Chemother, doi:10.1093/jac/dkaa418
Singh, Parida, Lingaraju, Kesavan, Kumar et al., Drug repurposing approach to fight COVID-19, Pharmacol Rep, doi:10.1007/s43440-020-00155-6
Walmsley, Antela, Clumeck, Duiculescu, Gutiérrez, Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection, N Engl J Med, doi:10.1056/NEJMoa1215541
Wang, Guan, COVID-19 drug repurposing: a review of computational screening methods, clinical trials, and protein interaction assays, Med Res Rev, doi:10.1002/med.21728
Wang, Guo, Liu, Wang, Rao et al., Endocytosis of the receptor-binding domain of SARS-CoV spike protein together with virus receptor ACE2, Virus Res, doi:10.1016/j.virusres.2008.03.004
Wang, Xu, Wang, Hong, Zhang et al., Conformational dynamics of the Beta and Kappa SARS-CoV-2 spike proteins and their complexes with ACE2 receptor revealed by cryo-EM, Nat Commun, doi:10.1038/s41467-021-27350-0
Wrapp, Wang, Corbett, Goldsmith, Hsieh et al., Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation, Science, doi:10.1126/science.abb2507
Xia, Zhang, Wang, Wang, Yang et al., Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30831-8
Xue, Li, Xie, Wang, Review of drug repositioning approaches and resources, Int J Biol Sci, doi:10.7150/ijbs.24612
Ye, Xu, Rong, Xu, Liu et al., Delivery of infection from asymptomatic carriers of COVID-19 in a familial cluster, International Journal of Infectious Diseases, doi:10.1016/j.ijid.2020.03.042
Ying, Ebrahimi, Keivan, Khoshnam, Salahi et al., miRNAs; a novel strategy for the treatment of COVID-19, Cell Biol Int, doi:10.1002/cbin.11653
Yu, Chen, Lan, Shen, Li, Computational screening of antagonists against the SARS-CoV-2 (COVID-19) coronavirus by molecular docking, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.106012
Zheng, Ma, Zhang, Xie, COVID-19 and the cardiovascular system, Nat Rev Cardiol, doi:10.1038/s41569-020-0360-5
Zheng, Shao, Chen, Zhang, Wang et al., Realworld effectiveness of COVID-19 vaccines: a literature review and meta-analysis, Int J Infect Dis, doi:10.1016/j.ijid.2021.11.009
DOI record:
{
"DOI": "10.34172/bi.29952",
"ISSN": [
"2228-5652",
"2228-5660"
],
"URL": "http://dx.doi.org/10.34172/bi.29952",
"abstract": "<jats:p>Introduction: Drug repurposing as a low-cost, time-saving, and often less risky strategy has been attractive for the treatment of coronavirus disease 2019 (COVID-19) during the pandemic. This trial aimed to evaluate the effectiveness of dolutegravir, an HIV-1 integrase inhibitor, in admitted patients with moderate COVID-19. Methods: This study was a randomized, double-blind, placebo-controlled clinical trial assessing the efficacy of dolutegravir in adults admitted to a hospital in Ghaemshahr, Mazandaran Province, Iran. Patients aged 18-80 years with early symptoms of moderate COVID-19, which was confirmed based on reverse transcription polymerase chain reaction (RT-PCR) and/or chest computed tomography (CT) scan, were considered to be included in this study. Patients were randomly assigned in a 1:1 ratio to receive 50 mg dolutegravir plus the standard treatment regimen or the same value of placebo plus the standard treatment regimen, daily for 7 days. The standard treatment regimen was remdesivir 200 mg on day 1 followed by 100 mg for five days or until discharge. The primary endpoint was recovery 10 days after the beginning of the study. Results: Between August 22 and October 23, 2021, of 120 patients who were enrolled, 93 patients were randomly assigned to receive 50 mg dolutegravir (n = 46) or the placebo regimen (n = 47). No significant difference was observed between the two intervention groups based on the obtained results including frequency of respiratory modes during the first five days of admission, respiratory rate, and O2 saturation during six time periods. Conclusion: The results showed that in adult patients admitted to the hospital with moderate COVID-19, treatment with dolutegravir was not associated with improvement in clinical recovery. Larger randomized trials are required to provide more robust evidence about the effectiveness of dolutegravir.</jats:p>",
"assertion": [
{
"label": "Journal Owner",
"name": "journal_owner",
"value": "Tabriz University of Medical Sciences"
},
{
"label": "Journal Publisher",
"name": "journal_publisher",
"value": "Tabriz University of Medical Sciences"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "2023-06-25"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2024-02-06"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2024-06-26"
}
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-6441-139X",
"affiliation": [
{
"name": "Department of Clinical Pharmacy, School of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran"
}
],
"authenticated-orcid": true,
"family": "Abbaspour Kasgari",
"given": "Hamideh",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-0222-9920",
"affiliation": [
{
"name": "Education Development Center, Mazandaran University of Medical Sciences, Sari, Iran"
}
],
"authenticated-orcid": true,
"family": "Moradi",
"given": "Siavash",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Antimicrobial Resistance Research Center, Mazandaran University of Medical Sciences, Sari, Iran"
}
],
"family": "Alikhani",
"given": "Ahmad",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-0404-5949",
"affiliation": [
{
"name": "Department of Life Science Engineering, Faculty of New Sciences and Technologies, University of Tehran, Tehran, Iran"
},
{
"name": "Department of Medical Nanotechnology, Faculty of Advanced Technologies in Medicine, Mazandaran University of Medical Sciences, Sari, Iran"
}
],
"authenticated-orcid": true,
"family": "Ahmadian",
"given": "Nasim",
"sequence": "additional"
}
],
"container-title": "BioImpacts",
"container-title-short": "Bioimpacts",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"bi.tbzmed.ac.ir"
]
},
"created": {
"date-parts": [
[
2024,
8,
19
]
],
"date-time": "2024-08-19T08:22:10Z",
"timestamp": 1724055730000
},
"deposited": {
"date-parts": [
[
2024,
8,
19
]
],
"date-time": "2024-08-19T08:22:12Z",
"timestamp": 1724055732000
},
"indexed": {
"date-parts": [
[
2025,
2,
21
]
],
"date-time": "2025-02-21T21:45:57Z",
"timestamp": 1740174357604,
"version": "3.37.3"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
6,
26
]
]
},
"language": "en",
"link": [
{
"URL": "https://bi.tbzmed.ac.ir/Inpress/bi-29952.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://bi.tbzmed.ac.ir/Inpress/bi-29952.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "20123",
"original-title": [],
"prefix": "10.34172",
"published": {
"date-parts": [
[
2024,
6,
26
]
]
},
"published-online": {
"date-parts": [
[
2024,
6,
26
]
]
},
"publisher": "Maad Rayan Publishing Company",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://bi.tbzmed.ac.ir/Inpress/bi-29952"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Effectiveness of dolutegravir in moderate severity COVID-19 patients: A single-center, randomized, double-blind, placebo-controlled trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.34172/crossmark_policy"
}