Feb 23 |
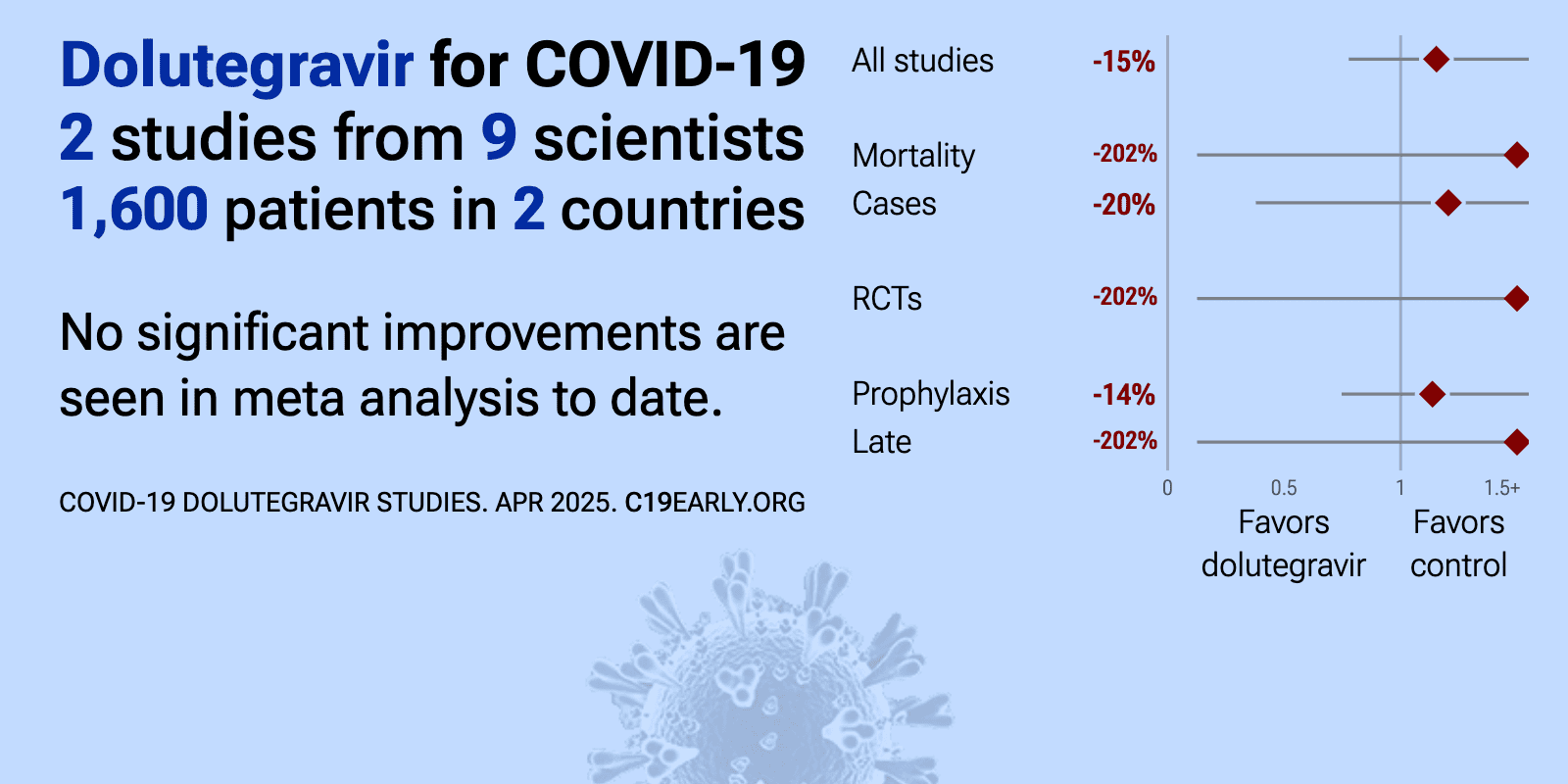
Meta-analysis of dolutegravir studies | |
| Meta-analysis of dolutegravir studies | ||
Jun 26 2024 |
et al., BioImpacts, doi:10.34172/bi.29952 | Effectiveness of dolutegravir in moderate severity COVID-19 patients: A single-center, randomized, double-blind, placebo-controlled trial |
| 202% higher mortality (p=0.49) and 5% lower need for oxygen therapy (p=1). RCT 93 hospitalized patients with moderate COVID-19 showing no significant difference in clinical outcomes with dolutegravir treatment compared to placebo. | ||
Apr 13 2023 |
et al., AIDS, doi:10.1097/QAD.0000000000003577 | No association between use of tenofovir disoproxil fumarate, etravirine, or integrase-strand transfer inhibitors and acquisition or severe outcomes of SARS-CoV-2 infection in people with HIV in the Netherlands |
| 14% higher combined mortality/hospitalization (p=0.54) and 20% more cases (p=0.75). Analysis of two Dutch cohorts with HIV showing no association between tenofovir disoproxil fumarate (TDF), etravirine (ETR), or integrase-strand transfer inhibitors (INSTIs) use and either the risk of incident SARS-CoV-2 infection or seve.. | ||