The efficacy of paxlovid in elderly patients infected with SARS-CoV-2 omicron variants: Results of a non-randomized clinical trial
et al., Frontiers in Medicine, doi:10.3389/fmed.2022.980002, ChiCTR2200060700, Sep 2022
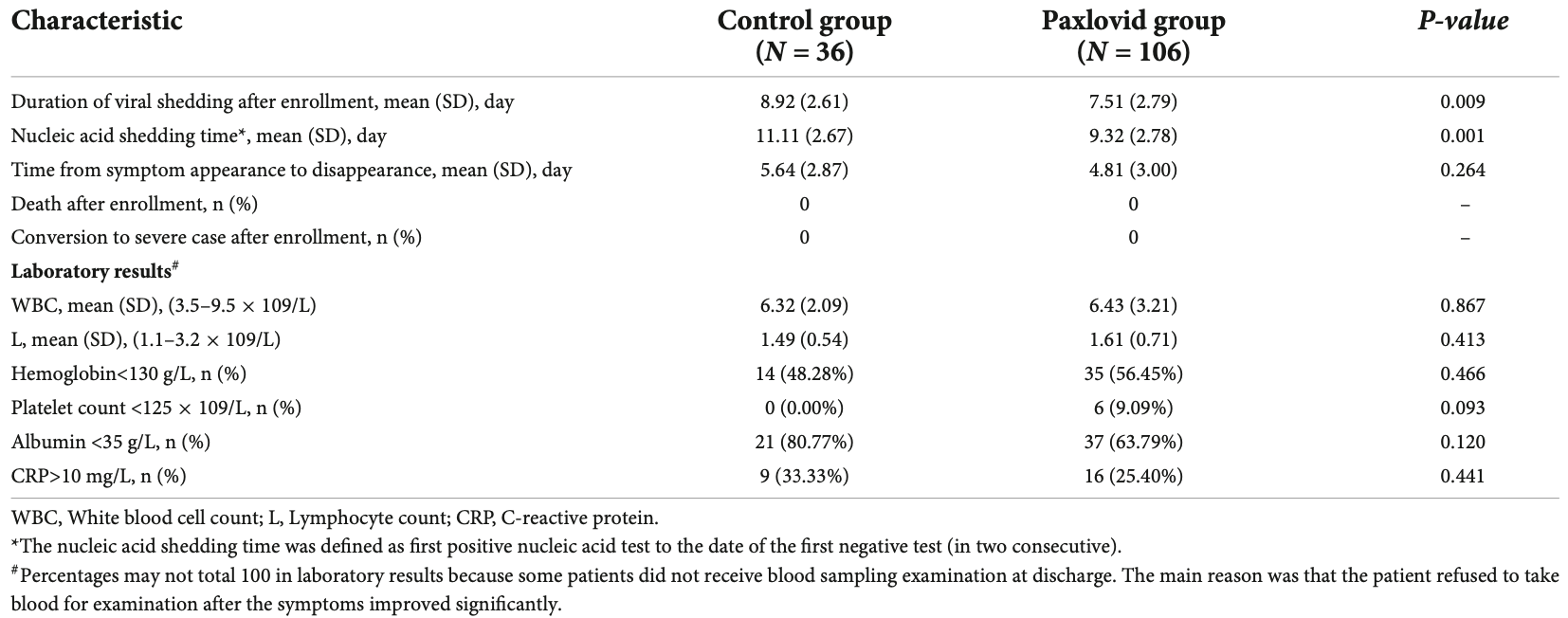
Retrospective 106 paxlovid and 36 control patients in China, showing faster viral clearance with treatment.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments18.
|
recovery time, 14.7% lower, relative time 0.85, p = 0.26, treatment mean 4.81 (±3.0) n=106, control mean 5.64 (±2.87) n=36.
|
|
time to viral-, 15.8% lower, relative time 0.84, p = 0.009, treatment mean 7.51 (±2.79) n=106, control mean 8.92 (±2.61) n=36, viral shedding after enrollment.
|
|
time to viral-, 16.1% lower, relative time 0.84, p = 0.001, treatment mean 9.32 (±2.78) n=106, control mean 11.11 (±2.67) n=36, first positive to first of two negative tests.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
16.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Zhong et al., 6 Sep 2022, retrospective, China, peer-reviewed, 12 authors, study period 24 April, 2022 - 28 May, 2022, trial ChiCTR2200060700.
Contact: snailliyi@163.com, zhangwench88@hotmail.com, zhuchuanlong@jsph.org.cn.
The efficacy of paxlovid in elderly patients infected with SARS-CoV-2 omicron variants: Results of a non-randomized clinical trial
Frontiers in Medicine, doi:10.3389/fmed.2022.980002
The efficacy of Paxlovid in elderly patients infected with SARS-CoV-2 omicron variants: Results of a non-randomized clinical trial.
Ethics statement The studies involving human participants were reviewed and approved by the Ethics Committee of the Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine (No. SH9H-2022-T112-2). Moreover, it was registered at the Chinese Clinical Trial Registry (ChiCTR2200060700). The patients/participants provided their written informed consent to participate in this study.
Author contributions WJZ, XJ, XY, ZD, WW, ZS, WCZ, LC, and YL collected the epidemiological and clinical data. WCZ and YL were responsible for enrollment, clinical monitoring, funding, study conception and design, and revising and submitting the final manuscript. XY, ZD, XJ, and YL were responsible for the distribution and storage of medicines. WJZ, TF, XN, LC, CZ, YL, and WCZ were responsible for statistical data. WJZ, XJ, XY, TF, CZ, and YL drafted the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the..
References
Carreño, Alshammary, Tcheou, Singh, Raskin et al., Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron, Nature, doi:10.1038/d41586-021-03846-z
Chilamakuri, Agarwal, COVID-19: characteristics and therapeutics, Cells, doi:10.3390/cells10020206
Dejnirattisai, Huo, Zhou, Zahradník, Supasa et al., SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses, Cell
Fan, Li, Zhang, Wan, Zhang et al., SARS-CoV-2 Omicron variant: recent progress and future perspectives, Signal Transduct Target Ther, doi:10.1038/s41392-022-00997-x
Fiolet, Kherabi, Macdonald, Ghosn, Peiffer-Smadja, Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review, Clin Microbiol Infect, doi:10.1016/j.cmi.2021.10.005
Garcia-Beltran, Denis, Hoelzemer, Lam, Nitido et al., mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant, doi:10.1101/2021.12.14.21267755
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Hirabara, Serdan, Gorjao, Masi, Pithon-Curi et al., SARS-COV-2 variants: differences and potential of immune evasion, Front Cell Infect Microbiol, doi:10.1016/S2213-2600(21)00559-2
Hu, Guo, Zhou, Shi, Characteristics of SARS-CoV-2 and COVID-19
Hung, Lee, Chiu, Lee, Tsai et al., Oral nirmatrelvir/ritonavir therapy for COVID-19: the dawn in the dark?, Antibiotics, doi:10.3390/antibiotics11020220
Jose, Manuel, COVID-19 cytokine storm: the interplay between inflammation and coagulation, Lancet Respir Med, doi:10.1016/S2213-2600(20)30216-2
Kawaoka, Uraki, Kiso, Iida, Imai et al., Characterization and antiviral susceptibility of SARS-CoV-2 Omicron/BA.2, Res Sq, doi:10.21203/rs.3.rs-1375091/v1
Ledford, How severe are Omicron infections?, Nature, doi:10.1038/d41586-021-03794-8
Marzi, Vakil, Bahmanyar, Zarenezhad, Paxlovid: mechanism of action, synthesis, and in silico study, Biomed Res Int, doi:10.1155/2022/7341493
Mistry, Barmania, Mellet, Peta, Strydom et al., SARS-CoV-2 variants, vaccines, and host immunity, Front Immunol, doi:10.3389/fimmu.2021.809244
Rössler, Riepler, Bante, Laer, Kimpel, SARS-CoV-2 Omicron variant neutralization in serum from vaccinated and convalescent persons, N Engl J Med, doi:10.1056/NEJMc2119236
Shen, Lin, Zhang, Li, Wang, Signals of significantly increased vaccine breakthrough, decreased hospitalization rates, and less severe disease in patients with coronavirus disease 2019 caused by the Omicron variant of severe acute respiratory syndrome coronavirus 2 in Houston, Texas, Emerg Microb Infect, doi:10.1016/j.ajpath.2022.01.007
Takashita, Kinoshita, Yamayoshi, Sakai-Tagawa, Fujisaki et al., Efficacy of antiviral agents against the SARS-CoV-2 Omicron subvariant BA.2, N Engl J Med, doi:10.1056/NEJMc2201933
Wang, Cheng, Zhang, Zhang, Chen, Shanghai's life-saving efforts against the current Omicron wave of the COVID-19 pandemic, J Med Virol, doi:10.1016/S0140-6736(22)00838-8
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet, doi:10.1016/S0140-6736(20)30566-3
DOI record:
{
"DOI": "10.3389/fmed.2022.980002",
"ISSN": [
"2296-858X"
],
"URL": "http://dx.doi.org/10.3389/fmed.2022.980002",
"abstract": "<jats:sec><jats:title>Objective</jats:title><jats:p>To evaluate the efficacy of Paxlovid in treating Chinese elder patients infected with SARS-CoV-2 omicron variants.</jats:p></jats:sec><jats:sec><jats:title>Materials and methods</jats:title><jats:p>We performed a non-randomized, controlled trial in Shanghai, China. Participants infected with SARS-CoV-2 omicron variants were enrolled. All patients were divided into the Paxlovid group or the control group according to the Chinese guideline (version 9). The nucleic acid shedding time was the primary endpoint.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>According to the inclusion criteria, 142 patients infected with omicron variants were enrolled, 36 patients who did not receive paxlovid were assigned to the control group, and 106 were in the Paxlovid group. The baseline characteristics were similar in either group. No significant difference in BMI, age, time from onset to patient enrollment, the severity on first admission, vaccination status, comorbidity, first symptoms, and laboratory results were recorded. Compared to the control group, participants in the Paxlovid group had a shorter viral shedding time [11.11 (2.67) vs. 9.32 (2.78), <jats:italic>P</jats:italic> = 0.001].</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>In Chinese elder patients infected with the variant of SARS-CoV-2 omicron, our data suggest that Paxlovid can significantly reduce the nucleic acid shedding time.</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fmed.2022.980002"
],
"author": [
{
"affiliation": [],
"family": "Zhong",
"given": "Weijie",
"sequence": "first"
},
{
"affiliation": [],
"family": "Jiang",
"given": "Xiufeng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Xiaosheng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Feng",
"given": "Tiantong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duan",
"given": "Zhixin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Wei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sun",
"given": "Zhaoliang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Lingyan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nie",
"given": "Xin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhu",
"given": "Chuanlong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Wenchuan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Yi",
"sequence": "additional"
}
],
"container-title": "Frontiers in Medicine",
"container-title-short": "Front. Med.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2022,
9,
6
]
],
"date-time": "2022-09-06T04:49:05Z",
"timestamp": 1662439745000
},
"deposited": {
"date-parts": [
[
2022,
9,
6
]
],
"date-time": "2022-09-06T04:49:08Z",
"timestamp": 1662439748000
},
"indexed": {
"date-parts": [
[
2022,
9,
6
]
],
"date-time": "2022-09-06T05:14:07Z",
"timestamp": 1662441247807
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
9,
6
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
6
]
],
"date-time": "2022-09-06T00:00:00Z",
"timestamp": 1662422400000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2022.980002/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2022,
9,
6
]
]
},
"published-online": {
"date-parts": [
[
2022,
9,
6
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1038/s41579-020-00459-7",
"article-title": "Characteristics of SARS-CoV-2 and COVID-19.",
"author": "Hu",
"doi-asserted-by": "publisher",
"first-page": "141",
"journal-title": "Nat Rev Microbiol.",
"key": "B1",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(20)30216-2",
"article-title": "COVID-19 cytokine storm: the interplay between inflammation and coagulation.",
"author": "Jose",
"doi-asserted-by": "publisher",
"first-page": "e46",
"journal-title": "Lancet Respir Med.",
"key": "B2",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.cmi.2021.10.005",
"article-title": "Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review.",
"author": "Fiolet",
"doi-asserted-by": "publisher",
"first-page": "202",
"journal-title": "Clin Microbiol Infect.",
"key": "B3",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1038/s41392-022-00997-x",
"article-title": "SARS-CoV-2 Omicron variant: recent progress and future perspectives.",
"author": "Fan",
"doi-asserted-by": "publisher",
"journal-title": "Signal Transduct Target Ther.",
"key": "B4",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2021.809244",
"article-title": "SARS-CoV-2 variants, vaccines, and host immunity.",
"author": "Mistry",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol.",
"key": "B5",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.3389/fcimb.2021.781429",
"article-title": "SARS-COV-2 variants: differences and potential of immune evasion.",
"author": "Hirabara",
"doi-asserted-by": "publisher",
"journal-title": "Front Cell Infect Microbiol.",
"key": "B6",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00559-2",
"article-title": "Omicron variant and booster COVID-19 vaccines.",
"author": "Burki",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Respir Med.",
"key": "B7",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1038/d41586-021-03794-8",
"article-title": "How severe are Omicron infections?",
"author": "Ledford",
"doi-asserted-by": "publisher",
"first-page": "577",
"journal-title": "Nature.",
"key": "B8",
"volume": "600",
"year": "2021"
},
{
"DOI": "10.1002/jmv.27516",
"article-title": "Sequence analysis of the emerging SARS-CoV-2 variant Omicron in South Africa.",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "1728",
"journal-title": "J Med Virol.",
"key": "B9",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)00838-8",
"article-title": "Shanghai’s life-saving efforts against the current Omicron wave of the COVID-19 pandemic.",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "2011",
"journal-title": "Lancet.",
"key": "B10",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.21203/rs.3.rs-1375091/v1",
"article-title": "Characterization and antiviral susceptibility of SARS-CoV-2 Omicron/BA.2.",
"author": "Kawaoka",
"doi-asserted-by": "publisher",
"journal-title": "Res Sq",
"key": "B11",
"year": "2022"
},
{
"DOI": "10.1080/22221751.2022.2078230",
"article-title": "An open, prospective cohort study of VV116 in Chinese participants infected with SARS-CoV-2 Omicron variants.",
"author": "Shen",
"doi-asserted-by": "publisher",
"first-page": "1518",
"journal-title": "Emerg Microb Infect.",
"key": "B12",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.4081/ejtm.2022.10355",
"article-title": "The outbreak of the SARS-CoV-2 Omicron variant make imperative the adoption of telerehabilitation in the Bulgarian health care system.",
"author": "Papathanasiou",
"doi-asserted-by": "publisher",
"journal-title": "Eur J Trans Myol.",
"key": "B13",
"volume": "32",
"year": "2022"
},
{
"DOI": "10.1016/j.ajpath.2022.01.007",
"article-title": "Signals of significantly increased vaccine breakthrough, decreased hospitalization rates, and less severe disease in patients with coronavirus disease 2019 caused by the Omicron variant of severe acute respiratory syndrome coronavirus 2 in Houston, Texas.",
"author": "Christensen",
"doi-asserted-by": "publisher",
"first-page": "642",
"journal-title": "Am J Pathol.",
"key": "B14",
"volume": "192",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2021.12.046",
"article-title": "SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses.",
"author": "Dejnirattisai",
"doi-asserted-by": "crossref",
"first-page": "467",
"journal-title": "Cell.",
"key": "B15",
"volume": "185",
"year": "2022"
},
{
"DOI": "10.1038/d41586-021-03846-z",
"article-title": "Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron.",
"author": "Carreño",
"doi-asserted-by": "publisher",
"first-page": "682",
"journal-title": "Nature.",
"key": "B16",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.1101/2021.12.14.21267755",
"article-title": "mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant.",
"author": "Garcia-Beltran",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "B17",
"year": "2021"
},
{
"DOI": "10.1056/NEJMc2119236",
"article-title": "SARS-CoV-2 Omicron variant neutralization in serum from vaccinated and convalescent persons.",
"author": "Rössler",
"doi-asserted-by": "publisher",
"first-page": "698",
"journal-title": "N Engl J Med.",
"key": "B18",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1155/2022/7341493",
"article-title": "Paxlovid: mechanism of action, synthesis, and in silico study.",
"author": "Marzi",
"doi-asserted-by": "publisher",
"journal-title": "Biomed Res Int.",
"key": "B19",
"volume": "2022",
"year": "2022"
},
{
"DOI": "10.3390/antibiotics11020220",
"article-title": "Oral nirmatrelvir/ritonavir therapy for COVID-19: the dawn in the dark?",
"author": "Hung",
"doi-asserted-by": "publisher",
"journal-title": "Antibiotics.",
"key": "B20",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2201933",
"article-title": "Efficacy of antiviral agents against the SARS-CoV-2 Omicron subvariant BA.2.",
"author": "Takashita",
"doi-asserted-by": "publisher",
"first-page": "1475",
"journal-title": "N Engl J Med.",
"key": "B21",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study.",
"author": "Zhou",
"doi-asserted-by": "publisher",
"first-page": "1054",
"journal-title": "Lancet.",
"key": "B22",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.3390/cells10020206",
"article-title": "COVID-19: characteristics and therapeutics.",
"author": "Chilamakuri",
"doi-asserted-by": "publisher",
"journal-title": "Cells.",
"key": "B23",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19.",
"author": "Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"journal-title": "N Engl J Med.",
"key": "B24",
"volume": "386",
"year": "2022"
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2022.980002/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "The efficacy of paxlovid in elderly patients infected with SARS-CoV-2 omicron variants: Results of a non-randomized clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "9"
}