Tocilizumab combined with favipiravir in the treatment of COVID-19: A multicenter trial in a small sample size
et al., Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2020.110825, Sep 2020
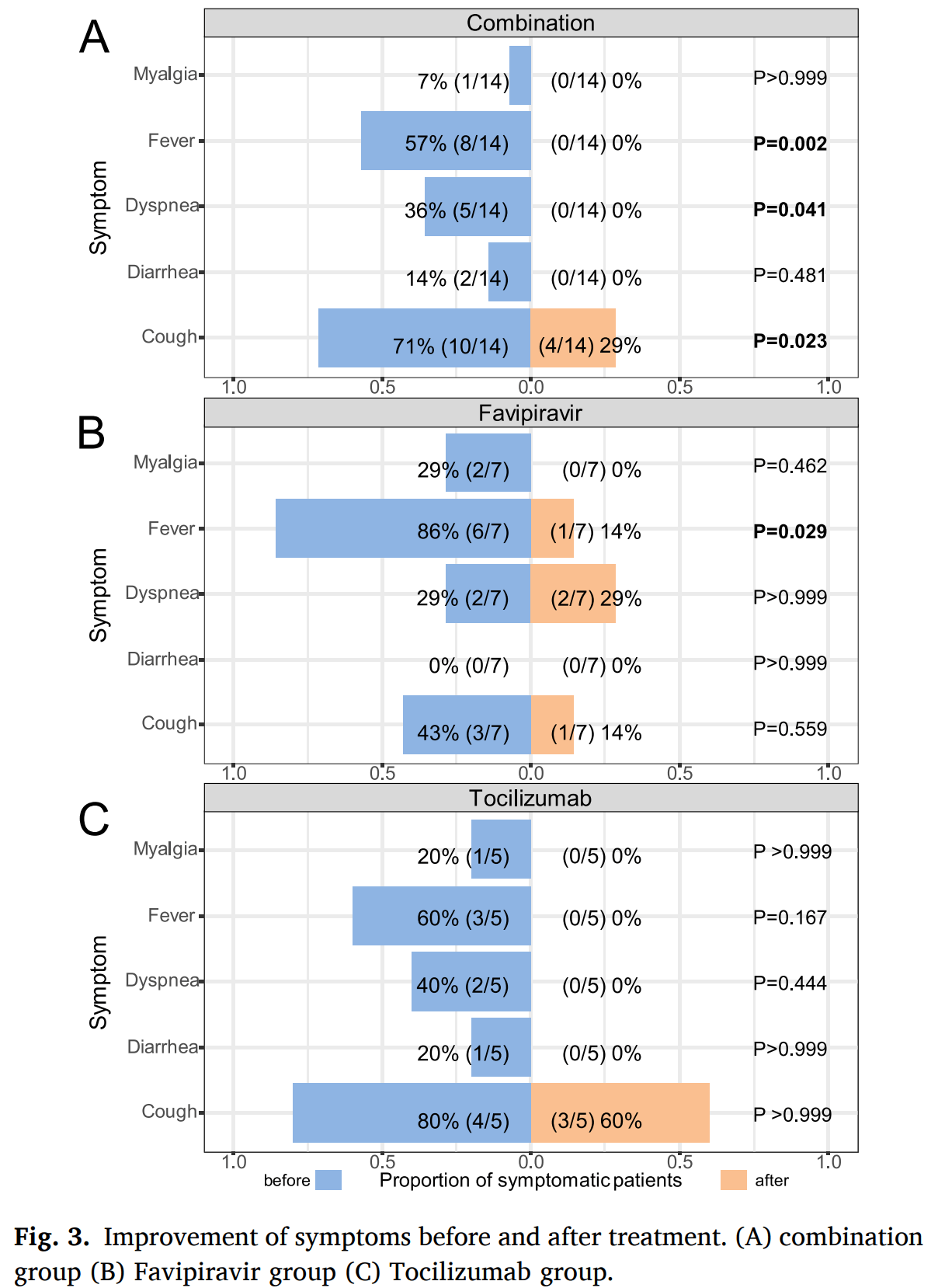
Small study with 14 combined favipiravir/tocilizumab, 7 favipiravir, and 5 tocilizumab patients suggesting that tocilizumab combined with or without favipiravir can improve pulmonary inflammation and inhibit progression.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Zhao et al., 30 Sep 2020, peer-reviewed, 12 authors.
Tocilizumab combined with favipiravir in the treatment of COVID-19: A multicenter trial in a small sample size
Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2020.110825
Background: Since December 2019, COVID-19 has spread to almost every corner of the world. In theory, tocilizumab and favipiravir are considered to be reliable drugs for the treatment of COVID-19 with elevated IL-6. We aimed to assess the efficacy and safety of tocilizumab combined with favipiravir in patients with COVID-19. Methods: This was a multicenter trial in adults with COVID-19. Patients were randomly assigned (3:1:1) to a 14day combination of favipiravir combined with tocilizumab (combination group), favipiravir, and tocilizumab. The primary outcome was the cumulative lung lesion remission rate (lung CT examination indicated absorption of lung inflammation). Results: Between Feb 2 and March 15, 2020, 26 patients were recruited; 14 were randomly assigned to the combination group, 7 were assigned to the favipiravir group and 5 were assigned to the tocilizumab group. The cumulative lung lesion remission rate at day 14 was significantly higher in combination group as compared with favipiravir group (P = 0.019, HR 2.66 95 % CI [1.08-6.53]). And there was also a significant difference between tocilizumab and favipivavir (P = 0.034, HR 3.16, 95 % CI 0.62-16.10). In addition, there was no significant difference between the combination group and the tocilizumab group (P = 0.575, HR 1.28 95 %CI 0.39-4.23). Furthermore, combined therapy can also significantly relieve clinical symptoms and help blood routine to return to normal. No serious adverse events were reported. Conclusion: Tocilizumab combined with or without favipiravir can effectively improve the pulmonary inflammation of COVID-19 patients and inhibit the deterioration of the disease.
Ethical approval and consent to participate This study has been approved by the Ethics Committee of Peking University First Hospital (2020-032) and has been registered in Chinese clinical trial registry (ChiCTR2000030096 and NCT04310228). All patients signed the informed consent form.
Consent for publication
Not applicable.
Authors' contributions Hong Zhao, Qi Zhu and Chi Zhang drafted the manuscript; Ming Wei, Yuhong Qin, Guilin Chen, Ke Wang, and Junhua Yu participated in the collection and arrangement of clinical cases; Chi Zhang and Jiawen Li participated in the creation of figures and tables; Zhao Wu, and Chi Zhang participated in the proofreading of this paper; Xianxiang Chen and Guiqiang Wang provided the overall principle and direction of the study. All authors read and approved the final manuscript.
Declaration of Competing Interest The authors report no declarations of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A. Supplementary data Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.biopha.2020.110825.
References
Cai, Experimental treatment with Favipiravir for COVID-19: an open-label control study
Chen, Management of cytokine release syndrome related to CAR-T cell therapy, Front. Med
Coperchini, The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system, Cytokine Growth Factor Rev
Della-Torre, Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study, Ann. Rheum. Dis
Emery, IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebocontrolled trial, Ann. Rheum. Dis
Furuta, Favipiravir (T-705), a novel viral RNA polymerase inhibitor, Antiviral Res
Genovese, Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to diseasemodifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study, Arthritis Rheum
Grupp, Chimeric antigen receptor-modified T cells for acute lymphoid leukemia, N. Engl. J. Med
Herold, Level of IL-6 predicts respiratory failure in hospitalized symptomatic COVID-19 patients, medRxiv
Hunter, Jones, IL-6 as a keystone cytokine in health and disease, Nat. Immunol
Jones, Jenkins, Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer, Nat. Rev. Immunol
Jones, The AMBITION trial: tocilizumab monotherapy for rheumatoid arthritis, Expert Rev. Clin. Immunol
Li, Early transmission dynamics in Wuhan, China, of novel coronavirusinfected pneumonia, N. Engl. J. Med
Liu, The role of interleukin-6 in monitoring severe case of coronavirus disease, EMBO Mol. Med
Neurath, Susetta Finotto, IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer, Cytokine Growth Factor Rev
Nishimoto, Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease, Blood
Peng-Jiao An, Zhu, Yang, Biochemical indicators of coronavirus disease 2019 exacerbation and the clinical implications, Pharmacol. Res
Ramiro, Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study, Ann. Rheum. Dis
Smolen, Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebocontrolled, randomised trial, Lancet
Somers, Tocilizumab for treatment of mechanically ventilated patients with COVID-19, Clin. Infect. Dis
Wang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Winkler, Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8), Blood
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention, JAMA
Xu, Effective treatment of severe COVID-19 patients with tocilizumab, Proc. Natl. Acad. Sci
Zhang, Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality, Int. J. Antimicrob. Agents
Zhou, Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients, Sci. Rev
DOI record:
{
"DOI": "10.1016/j.biopha.2020.110825",
"ISSN": [
"0753-3322"
],
"URL": "http://dx.doi.org/10.1016/j.biopha.2020.110825",
"alternative-id": [
"S0753332220310180"
],
"article-number": "110825",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Tocilizumab combined with favipiravir in the treatment of COVID-19: A multicenter trial in a small sample size"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Biomedicine & Pharmacotherapy"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.biopha.2020.110825"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2020 Published by Elsevier Masson SAS."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-8069-9901",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zhao",
"given": "Hong",
"sequence": "first"
},
{
"affiliation": [],
"family": "Zhu",
"given": "Qi",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5989-0736",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zhang",
"given": "Chi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Jiawen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wei",
"given": "Ming",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Qin",
"given": "Yuhong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Guilin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Ke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yu",
"given": "Junhua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wu",
"given": "Zhao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Xianxiang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Guiqiang",
"sequence": "additional"
}
],
"container-title": "Biomedicine & Pharmacotherapy",
"container-title-short": "Biomedicine & Pharmacotherapy",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"em-consulte.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2020,
9,
30
]
],
"date-time": "2020-09-30T01:24:58Z",
"timestamp": 1601429098000
},
"deposited": {
"date-parts": [
[
2024,
1,
22
]
],
"date-time": "2024-01-22T17:31:08Z",
"timestamp": 1705944668000
},
"funder": [
{
"award": [
"2020YFC0844100",
"2020YFC0846800"
],
"name": "Chinese COVID-19 scientific research emergency project"
},
{
"award": [
"2017ZX10203202",
"2013ZX10002005"
],
"name": "China Mega-Project for Infectious Diseases"
},
{
"award": [
"2016ZX09101065"
],
"name": "China Mega-Project for Innovative Drugs"
}
],
"indexed": {
"date-parts": [
[
2024,
5,
14
]
],
"date-time": "2024-05-14T09:07:00Z",
"timestamp": 1715677620564
},
"is-referenced-by-count": 74,
"issued": {
"date-parts": [
[
2021,
1
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
1,
1
]
],
"date-time": "2021-01-01T00:00:00Z",
"timestamp": 1609459200000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
9,
28
]
],
"date-time": "2020-09-28T00:00:00Z",
"timestamp": 1601251200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0753332220310180?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0753332220310180?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "110825",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
1
]
]
},
"published-print": {
"date-parts": [
[
2021,
1
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMoa2001316",
"article-title": "Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "1199",
"issue": "13",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.biopha.2020.110825_bib0005",
"volume": "382",
"year": "2020"
},
{
"key": "10.1016/j.biopha.2020.110825_bib0010",
"unstructured": "http://2019ncov.chinacdc.cn/2019-nCoV/global.html (Accessed 28 May 2020)."
},
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "1239",
"issue": "13",
"journal-title": "JAMA",
"key": "10.1016/j.biopha.2020.110825_bib0015",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105954",
"article-title": "Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "105954",
"issue": "5",
"journal-title": "Int. J. Antimicrob. Agents",
"key": "10.1016/j.biopha.2020.110825_bib0020",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1016/j.phrs.2020.104946",
"article-title": "Biochemical indicators of coronavirus disease 2019 exacerbation and the clinical implications",
"author": "An",
"doi-asserted-by": "crossref",
"first-page": "104946",
"journal-title": "Pharmacol. Res.",
"key": "10.1016/j.biopha.2020.110825_bib0025",
"year": "2020"
},
{
"DOI": "10.1016/j.cytogfr.2020.05.003",
"article-title": "The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system",
"author": "Coperchini",
"doi-asserted-by": "crossref",
"journal-title": "Cytokine Growth Factor Rev.",
"key": "10.1016/j.biopha.2020.110825_bib0030",
"year": "2020"
},
{
"article-title": "Level of IL-6 predicts respiratory failure in hospitalized symptomatic COVID-19 patients",
"author": "Herold",
"journal-title": "medRxiv",
"key": "10.1016/j.biopha.2020.110825_bib0035",
"year": "2020"
},
{
"article-title": "The role of interleukin-6 in monitoring severe case of coronavirus disease 2019",
"author": "Liu",
"journal-title": "EMBO Mol. Med.",
"key": "10.1016/j.biopha.2020.110825_bib0040",
"year": "2020"
},
{
"key": "10.1016/j.biopha.2020.110825_bib0045",
"unstructured": "Instruction of Tocilizumab https://www.gene.com/download/pdf/actemra_prescribing.pdf (Accessed 28 May 2020)."
},
{
"DOI": "10.1182/blood.V94.7.2217.419k02_2217_2224",
"article-title": "Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8)",
"author": "Winkler",
"doi-asserted-by": "crossref",
"first-page": "2217",
"issue": "7",
"journal-title": "Blood",
"key": "10.1016/j.biopha.2020.110825_bib0050",
"volume": "94",
"year": "1999"
},
{
"DOI": "10.1056/NEJMoa1215134",
"article-title": "Chimeric antigen receptor-modified T cells for acute lymphoid leukemia",
"author": "Grupp",
"doi-asserted-by": "crossref",
"first-page": "1509",
"issue": "16",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.biopha.2020.110825_bib0055",
"volume": "368",
"year": "2013"
},
{
"article-title": "Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients",
"author": "Zhou",
"journal-title": "Sci. Rev.",
"key": "10.1016/j.biopha.2020.110825_bib0060",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2005615117",
"article-title": "Effective treatment of severe COVID-19 patients with tocilizumab",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "10970",
"issue": "20",
"journal-title": "Proc. Natl. Acad. Sci.",
"key": "10.1016/j.biopha.2020.110825_bib0065",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2013.09.015",
"article-title": "Favipiravir (T-705), a novel viral RNA polymerase inhibitor",
"author": "Furuta",
"doi-asserted-by": "crossref",
"first-page": "446",
"issue": "2",
"journal-title": "Antiviral Res.",
"key": "10.1016/j.biopha.2020.110825_bib0070",
"volume": "100",
"year": "2013"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "269",
"issue": "3",
"journal-title": "Cell Res.",
"key": "10.1016/j.biopha.2020.110825_bib0075",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1016/j.eng.2020.03.007",
"article-title": "Experimental treatment with Favipiravir for COVID-19: an open-label control study",
"author": "Cai",
"doi-asserted-by": "crossref",
"journal-title": "Engineering",
"key": "10.1016/j.biopha.2020.110825_bib0080",
"year": "2020"
},
{
"key": "10.1016/j.biopha.2020.110825_bib0085",
"series-title": "Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia",
"year": "2020"
},
{
"DOI": "10.1038/s41577-018-0066-7",
"article-title": "Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer",
"author": "Jones",
"doi-asserted-by": "crossref",
"first-page": "773",
"issue": "12",
"journal-title": "Nat. Rev. Immunol.",
"key": "10.1016/j.biopha.2020.110825_bib0090",
"volume": "18",
"year": "2018"
},
{
"DOI": "10.1038/ni.3153",
"article-title": "IL-6 as a keystone cytokine in health and disease",
"author": "Hunter",
"doi-asserted-by": "crossref",
"first-page": "448",
"issue": "5",
"journal-title": "Nat. Immunol.",
"key": "10.1016/j.biopha.2020.110825_bib0095",
"volume": "16",
"year": "2015"
},
{
"DOI": "10.1016/j.cytogfr.2011.02.003",
"article-title": "IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer",
"author": "Neurath",
"doi-asserted-by": "crossref",
"first-page": "83",
"issue": "2",
"journal-title": "Cytokine Growth Factor Rev.",
"key": "10.1016/j.biopha.2020.110825_bib0100",
"volume": "22",
"year": "2011"
},
{
"DOI": "10.1136/ard.2008.092932",
"article-title": "IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial",
"author": "Emery",
"doi-asserted-by": "crossref",
"first-page": "1516",
"issue": "11",
"journal-title": "Ann. Rheum. Dis.",
"key": "10.1016/j.biopha.2020.110825_bib0105",
"volume": "67",
"year": "2008"
},
{
"DOI": "10.1002/art.23940",
"author": "Genovese",
"doi-asserted-by": "crossref",
"first-page": "2968",
"issue": "10",
"journal-title": "Arthritis Rheum.",
"key": "10.1016/j.biopha.2020.110825_bib0110",
"volume": "58",
"year": "2008"
},
{
"DOI": "10.1586/eci.10.2",
"article-title": "The AMBITION trial: tocilizumab monotherapy for rheumatoid arthritis",
"author": "Jones",
"doi-asserted-by": "crossref",
"first-page": "189",
"issue": "2",
"journal-title": "Expert Rev. Clin. Immunol.",
"key": "10.1016/j.biopha.2020.110825_bib0115",
"volume": "6",
"year": "2010"
},
{
"DOI": "10.1016/S0140-6736(08)60453-5",
"article-title": "Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial",
"author": "Smolen",
"doi-asserted-by": "crossref",
"first-page": "987",
"issue": "9617",
"journal-title": "Lancet",
"key": "10.1016/j.biopha.2020.110825_bib0120",
"volume": "371",
"year": "2008"
},
{
"DOI": "10.1182/blood-2008-05-155846",
"article-title": "Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti–IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease",
"author": "Nishimoto",
"doi-asserted-by": "crossref",
"first-page": "3959",
"issue": "10",
"journal-title": "Blood",
"key": "10.1016/j.biopha.2020.110825_bib0125",
"volume": "112",
"year": "2008"
},
{
"DOI": "10.1007/s11684-019-0714-8",
"article-title": "Management of cytokine release syndrome related to CAR-T cell therapy",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "610",
"issue": "5",
"journal-title": "Front. Med.",
"key": "10.1016/j.biopha.2020.110825_bib0130",
"volume": "13",
"year": "2019"
},
{
"DOI": "10.1136/annrheumdis-2020-218479",
"article-title": "Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study",
"author": "Ramiro",
"doi-asserted-by": "crossref",
"first-page": "1143",
"issue": "9",
"journal-title": "Ann. Rheum. Dis.",
"key": "10.1016/j.biopha.2020.110825_bib0135",
"volume": "79",
"year": "2020"
},
{
"article-title": "Tocilizumab for treatment of mechanically ventilated patients with COVID-19",
"author": "Somers",
"journal-title": "Clin. Infect. Dis.",
"key": "10.1016/j.biopha.2020.110825_bib0140",
"year": "2020"
},
{
"DOI": "10.1136/annrheumdis-2020-218122",
"article-title": "Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study",
"author": "Della-Torre",
"doi-asserted-by": "crossref",
"first-page": "1277",
"issue": "10",
"journal-title": "Ann. Rheum. Dis.",
"key": "10.1016/j.biopha.2020.110825_bib0145",
"volume": "79",
"year": "2020"
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0753332220310180"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Tocilizumab combined with favipiravir in the treatment of COVID-19: A multicenter trial in a small sample size",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "133"
}