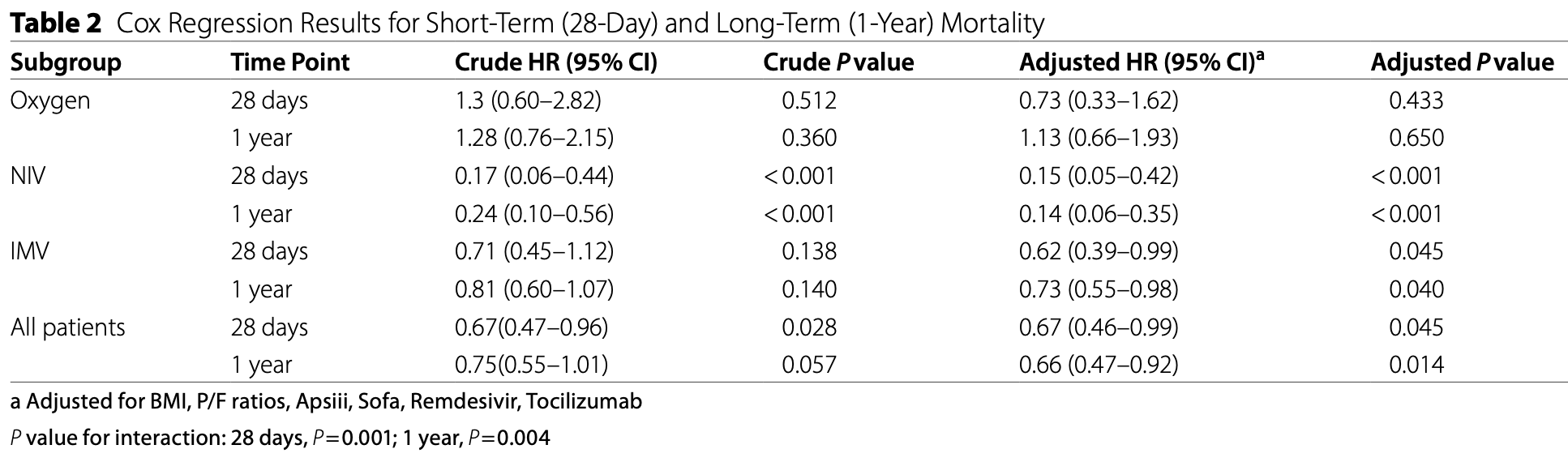
The impact of dexamethasone on short- and long-term mortality in hospitalized COVID-19 patients: a retrospective study
et al., BMC Infectious Diseases, doi:10.1186/s12879-024-10216-3, Nov 2024
Retrospective 576 hospitalized COVID-19 patients showing lower mortality with dexamethasone treatment.
Results are pending author clarification/correction due to critical internal inconsistencies. The respiratory support subgroup counts in the non-dexamethasone group sum to only 270 patients rather than the reported 288, indicating 18 patients are unaccounted for. Summing 28-day deaths across subgroups yields 66 deaths in the non-dexamethasone group versus 73 reported in Table 1, leaving 7 deaths unexplained. The propensity score matching appears to have failed, as 8 of 22 variables remain significantly imbalanced (p<0.05) after matching - including BMI, which was explicitly listed as a matching variable yet shows p = 0.004. The NIV subgroup reports an effect size about five times stronger than the RECOVERY trial and is driven by only 15 total deaths across severely imbalanced groups (n=18 vs n=79). A p-value inconsistency exists between Table 2 (p for interaction = 0.001) and the discussion (p = 0.011).
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This study is excluded in meta-analysis:
multiple potential data reliability issues.
|
risk of death, 34.0% lower, HR 0.66, p = 0.01, treatment 288, control 288, propensity score matching, day 365.
|
|
risk of death, 33.0% lower, HR 0.67, p = 0.01, treatment 288, control 288, propensity score matching, day 28.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Zhao et al., 25 Nov 2024, retrospective, USA, peer-reviewed, 7 authors.
Contact: 18917683768@189.cn, chenyuanzhuo@tongji.edu.cn.
The impact of dexamethasone on short- and long-term mortality in hospitalized COVID-19 patients: a retrospective study
BMC Infectious Diseases, doi:10.1186/s12879-024-10216-3
Background Dexamethasone has been widely used in treating severe COVID-19 patients due to its antiinflammatory properties. However, its long-term impact on mortality remain unclear. Objective To evaluate the effect of dexamethasone on short-term (28-day) and long-term (1-year) mortality in hospitalized COVID-19 patients and to explore its efficacy across different respiratory support. Methods A retrospective cohort study was conducted using the MIMIC-IV (v3.0) database. A total of 576 confirmed COVID-19 patients were included, with 288 patients receiving dexamethasone and 288 not receiving it, matched by propensity scores. Survival analyses assessed the impact of dexamethasone on mortality, and subgroup analyses were performed based on the type of respiratory support received.
Results After propensity score matching, dexamethasone treatment was associated with reduced mortality at both 28 days (adjusted HR 0.67, 95% CI 0.46-0.99, P = 0.045) and 1 year (adjusted HR 0.66, 95% CI 0.47-0.92, P = 0.014). Subgroup analysis revealed differential treatment effects by respiratory support type (P for interaction = 0.001 at 28 days and 0.004 at 1 year). The survival benefit was most pronounced in patients receiving NIV (28-day adjusted HR 0.15, 95% CI 0.05-0.42, P < 0.001) and significant in those receiving IMV (28-day adjusted HR 0.62, 95% CI 0.39-0.99, P = 0.045), while no significant benefit was observed in patients receiving oxygen therapy alone.
Conclusion This retrospective study suggests that dexamethasone treatment was associated with reduced mortality in hospitalized COVID-19 patients, particularly in those receiving NIV or IMV. These findings add to the evidence supporting dexamethasone use in severe COVID-19 patients requiring respiratory support.
Supplementary Information The online version contains supplementary material available at h t t p s : / / d o i . o r g / 1 0 . 1 1 8 6 / s 1 2 8 7 9 -0 2 4 -1 0 2
Author contributions Jian Zhao, Hui Hua Jiang, Hong Hong Wan, Dan Liu, and Yi Zhao contributed equally to this work. They were responsible for the conception, design, and execution of the study, as well as data collection and analysis. Yan Qing Chen and Yuan Zhuo Chen supervised the project, provided critical revisions, and guided the writing of the manuscript. All authors read and approved the final version of the manuscript.
Declarations Ethics approval and consent to participate The MIMIC-IV database was approved by the Institutional Review Boards of Massachusetts Institute of Technology (Cambridge, MA) and Beth Israel Deaconess Medical Center (Boston, MA). Informed consent was obtained for the original data collection. The requirement for individual patient consent was waived as the project did not impact clinical care and all protected health information was de-identified.
Consent for publication Non-applicable.
Competing interests The authors declare no competing interests.
Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Alhazzani, Møller, Arabi, Loeb, Gong, Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19), Crit Care Med
Bahl, Johnson, Chen, Timing of corticosteroids impacts mortality in hospitalized COVID-19 patients, Intern Emerg Med
Fadel, Morrison, Vahia, Smith, Chaudhry et al., Early short-course corticosteroids in hospitalized patients with COVID-19, CLIN INFECT DIS
Group, Tw, Sterne, Murthy, Diaz et al., Association between Administration of Systemic Corticosteroids and Mortality among critically ill patients with COVID-19, JAMA
Horby, Lim, Emberson, Mafham, Bell et al., Effect of Dexamethasone in Hospitalized Patients with COVID-19 -Preliminary Report, medRxiv
Issak, Amin, Timing of corticosteroids in non-severe non-hospitalized COVID-19 patients: open-label, two-center, randomized controlled study (TICS-COV19 study), Korean J Intern Med
Johnson, Pollard, Shen, Lehman, Feng et al., MIMIC-III, a freely accessible critical care database, Sci Data
Kow, Ramachandram, Hasan, Intermediate-to high-dose dexamethasone versus low-dose dexamethasone in patients with COVID-19 requiring respiratory support: a systematic review and meta-analysis of randomized trials, Inflammopharmacol
Liu, Zhang, Dong, Li, Xu et al., Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome, J Clin Invest
Maskin, Bonelli, Olarte, Palizas, Velo et al., High-Versus Low-Dose Dexamethasone for the treatment of COVID-19-Related Acute Respiratory Distress Syndrome: a Multicenter, Randomized Open-label clinical trial, J Intensive Care Med
Popa-Fotea, Dexamethasone in Hospitalized Patients with Covid-19
Richards, Feldman, The use of corticosteroids for COVID-19 infection, Afr J Thorac Crit Care Med
Singh, Kukreja, Arora, Gill, Effectiveness of Dexamethasone as an Adjunct Drug in Treatment of Critical COVID-19 Patients: An Observational Single Cohort, JCDR, doi:10.7860/jcdr/2022/52669.16025
Sun, Lin, Ai, Zhang, The efficacy of antivirals, corticosteroids, and monoclonal antibodies as acute COVID-19 treatments in reducing the incidence of long COVID: a systematic review and meta-analysis, Clin Microbiol Infect, doi:10.1016/j.cmi.2024.07.006
Taboada, Rodríguez, Varela, Rodríguez, Abelleira et al., Effect of high versus low dose of dexamethasone on clinical worsening in patients hospitalised with moderate or severe COVID-19 pneumonia: an open-label, randomised clinical trial, Eur Respir J
Tomazini, Maia, Cavalcanti, Berwanger, Rosa et al., Effect of dexamethasone on days alive and Ventilator-Free in patients with moderate or severe Acute Respiratory Distress Syndrome and COVID-19, JAMA
Villar, Ferrando, Martínez, Ambrós, Muñoz et al., Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial, Lancet Respir Med
Ye, Li, Luo, Xu, Kasimu et al., Efficacy and safety of glucocorticoids in the treatment of COVID-19: a systematic review and meta-analysis of RCTs, Clin Exp Med
Zhang, Su, Wu, Qiao, Gao et al., Efficacy of different doses of corticosteroids in treating severe COVID-19 pneumonia, Virol J
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet
DOI record:
{
"DOI": "10.1186/s12879-024-10216-3",
"ISSN": [
"1471-2334"
],
"URL": "http://dx.doi.org/10.1186/s12879-024-10216-3",
"alternative-id": [
"10216"
],
"article-number": "1343",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "23 September 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "13 November 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "25 November 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The MIMIC-IV database was approved by the Institutional Review Boards of Massachusetts Institute of Technology (Cambridge, MA) and Beth Israel Deaconess Medical Center (Boston, MA). Informed consent was obtained for the original data collection. The requirement for individual patient consent was waived as the project did not impact clinical care and all protected health information was de-identified."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Non-applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare no competing interests."
},
{
"group": {
"label": "Clinical trial registration",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 5,
"value": "Non-applicable."
}
],
"author": [
{
"affiliation": [],
"family": "Zhao",
"given": "Jian",
"sequence": "first"
},
{
"affiliation": [],
"family": "Jiang",
"given": "Hui Hua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wan",
"given": "Hong Hong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Dan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhao",
"given": "Yi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Yan Qing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Yuan Zhuo",
"sequence": "additional"
}
],
"container-title": "BMC Infectious Diseases",
"container-title-short": "BMC Infect Dis",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
11,
25
]
],
"date-time": "2024-11-25T14:15:36Z",
"timestamp": 1732544136000
},
"deposited": {
"date-parts": [
[
2024,
11,
25
]
],
"date-time": "2024-11-25T15:02:33Z",
"timestamp": 1732546953000
},
"indexed": {
"date-parts": [
[
2024,
11,
26
]
],
"date-time": "2024-11-26T05:11:46Z",
"timestamp": 1732597906995,
"version": "3.28.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
11,
25
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
11,
25
]
],
"date-time": "2024-11-25T00:00:00Z",
"timestamp": 1732492800000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
11,
25
]
],
"date-time": "2024-11-25T00:00:00Z",
"timestamp": 1732492800000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-024-10216-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12879-024-10216-3/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-024-10216-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2024,
11,
25
]
]
},
"published-online": {
"date-parts": [
[
2024,
11,
25
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.46945/bpj.10.1.03.01",
"doi-asserted-by": "crossref",
"key": "10216_CR1",
"unstructured": "E. C. WHO Coronavirus Disease (COVID-19) dashboard. BPJ. 2020;10."
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"author": "F Zhou",
"doi-asserted-by": "publisher",
"first-page": "1054",
"journal-title": "Lancet",
"key": "10216_CR2",
"unstructured": "Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1101/2020.06.22.20137273",
"doi-asserted-by": "crossref",
"key": "10216_CR3",
"unstructured": "Horby P, Lim WS, Emberson J, Mafham M, Bell J, Linsell L et al. Effect of Dexamethasone in Hospitalized Patients with COVID-19 – Preliminary Report. medRxiv. 2020."
},
{
"DOI": "10.1001/jama.2020.17021",
"author": "BM Tomazini",
"doi-asserted-by": "publisher",
"first-page": "1307",
"journal-title": "JAMA",
"key": "10216_CR4",
"unstructured": "Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of dexamethasone on days alive and Ventilator-Free in patients with moderate or severe Acute Respiratory Distress Syndrome and COVID-19. JAMA. 2020;324:1307.",
"volume": "324",
"year": "2020"
},
{
"key": "10216_CR5",
"unstructured": "Popa-Fotea N. Dexamethasone in Hospitalized Patients with Covid-19. 2021."
},
{
"DOI": "10.1093/cid/ciaa601",
"author": "R Fadel",
"doi-asserted-by": "publisher",
"first-page": "2114",
"journal-title": "CLIN INFECT DIS",
"key": "10216_CR6",
"unstructured": "Fadel R, Morrison AR, Vahia A, Smith ZR, Chaudhry Z, Bhargava P, et al. Early short-course corticosteroids in hospitalized patients with COVID-19. CLIN INFECT DIS. 2020;71:2114–20.",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.17023",
"author": "JAC Sterne",
"doi-asserted-by": "publisher",
"first-page": "1330",
"journal-title": "JAMA",
"key": "10216_CR7",
"unstructured": "Group TWREA for C-19 TW, Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, et al. Association between Administration of Systemic Corticosteroids and Mortality among critically ill patients with COVID-19. JAMA. 2020;324:1330.",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.7196/AJTCCM.2020.v26i3.106",
"author": "GA Richards",
"doi-asserted-by": "publisher",
"first-page": "87",
"journal-title": "Afr J Thorac Crit Care Med",
"key": "10216_CR8",
"unstructured": "Richards GA, Feldman C. The use of corticosteroids for COVID-19 infection. Afr J Thorac Crit Care Med. 2020;26:87.",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(19)30417-5",
"author": "J Villar",
"doi-asserted-by": "publisher",
"first-page": "267",
"journal-title": "Lancet Respir Med",
"key": "10216_CR9",
"unstructured": "Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–76.",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1097/CCM.0000000000004363",
"author": "W Alhazzani",
"doi-asserted-by": "publisher",
"first-page": "e440",
"journal-title": "Crit Care Med",
"key": "10216_CR10",
"unstructured": "Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Crit Care Med. 2020;48:e440–69.",
"volume": "48",
"year": "2020"
},
{
"DOI": "10.1172/JCI140617",
"author": "J Liu",
"doi-asserted-by": "publisher",
"first-page": "6417",
"journal-title": "J Clin Invest",
"key": "10216_CR11",
"unstructured": "Liu J, Zhang S, Dong X, Li Z, Xu Q, Feng H, et al. Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J Clin Invest. 2020;130:6417–28.",
"volume": "130",
"year": "2020"
},
{
"DOI": "10.1038/sdata.2016.35",
"doi-asserted-by": "crossref",
"key": "10216_CR12",
"unstructured": "Johnson AEW, Pollard TJ, Shen L, Lehman LH, Feng M, Ghassemi M et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3."
},
{
"DOI": "10.7860/jcdr/2022/52669.16025",
"doi-asserted-by": "publisher",
"key": "10216_CR13",
"unstructured": "Singh AP, Kukreja S, Arora R, Gill MK. Effectiveness of Dexamethasone as an Adjunct Drug in Treatment of Critical COVID-19 Patients: An Observational Single Cohort. JCDR. 2022. https://doi.org/10.7860/jcdr/2022/52669.16025"
},
{
"DOI": "10.1007/s10238-024-01405-0",
"doi-asserted-by": "crossref",
"key": "10216_CR14",
"unstructured": "Ye X, Li Y, Luo F, Xu Z, Kasimu K, Wang J et al. Efficacy and safety of glucocorticoids in the treatment of COVID-19: a systematic review and meta-analysis of RCTs. Clin Exp Med. 2024;24."
},
{
"DOI": "10.1016/j.cmi.2024.07.006",
"author": "G Sun",
"doi-asserted-by": "publisher",
"journal-title": "Clin Microbiol Infect",
"key": "10216_CR15",
"unstructured": "Sun G, Lin K, Ai J, Zhang W. The efficacy of antivirals, corticosteroids, and monoclonal antibodies as acute COVID-19 treatments in reducing the incidence of long COVID: a systematic review and meta-analysis. Clin Microbiol Infect. 2024. https://doi.org/10.1016/j.cmi.2024.07.006",
"year": "2024"
},
{
"DOI": "10.3904/kjim.2022.232",
"author": "ER Issak",
"doi-asserted-by": "publisher",
"first-page": "207",
"journal-title": "Korean J Intern Med",
"key": "10216_CR16",
"unstructured": "Issak ER, Amin MM. Timing of corticosteroids in non-severe non-hospitalized COVID-19 patients: open-label, two-center, randomized controlled study (TICS-COV19 study). Korean J Intern Med. 2023;38:207–17.",
"volume": "38",
"year": "2023"
},
{
"DOI": "10.1007/s11739-021-02655-6",
"author": "A Bahl",
"doi-asserted-by": "publisher",
"first-page": "1593",
"journal-title": "Intern Emerg Med",
"key": "10216_CR17",
"unstructured": "Bahl A, Johnson S, Chen N-W. Timing of corticosteroids impacts mortality in hospitalized COVID-19 patients. Intern Emerg Med. 2021;16:1593–603.",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1186/s12985-024-02345-7",
"doi-asserted-by": "crossref",
"key": "10216_CR18",
"unstructured": "zhang G, Su L, Wu W, Qiao Q, Gao S, Zhang Y et al. Efficacy of different doses of corticosteroids in treating severe COVID-19 pneumonia. Virol J. 2024;21."
},
{
"DOI": "10.1177/08850666211066799",
"author": "LP Maskin",
"doi-asserted-by": "publisher",
"first-page": "491",
"journal-title": "J Intensive Care Med",
"key": "10216_CR19",
"unstructured": "Maskin LP, Bonelli I, Olarte GL, Palizas F, Velo AE, Lurbet MF, et al. High- Versus Low-Dose Dexamethasone for the treatment of COVID-19-Related Acute Respiratory Distress Syndrome: a Multicenter, Randomized Open-label clinical trial. J Intensive Care Med. 2021;37:491–9.",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1007/s10787-023-01251-8",
"author": "CS Kow",
"doi-asserted-by": "publisher",
"first-page": "2773",
"journal-title": "Inflammopharmacol",
"key": "10216_CR20",
"unstructured": "Kow CS, Ramachandram DS, Hasan SS. Intermediate- to high-dose dexamethasone versus low-dose dexamethasone in patients with COVID-19 requiring respiratory support: a systematic review and meta-analysis of randomized trials. Inflammopharmacol. 2023;31:2773–9.",
"volume": "31",
"year": "2023"
},
{
"DOI": "10.1183/13993003.02518-2021",
"author": "M Taboada",
"doi-asserted-by": "publisher",
"first-page": "2102518",
"journal-title": "Eur Respir J",
"key": "10216_CR21",
"unstructured": "Taboada M, Rodríguez N, Varela PM, Rodríguez MT, Abelleira R, González A, et al. Effect of high versus low dose of dexamethasone on clinical worsening in patients hospitalised with moderate or severe COVID-19 pneumonia: an open-label, randomised clinical trial. Eur Respir J. 2021;60:2102518.",
"volume": "60",
"year": "2021"
}
],
"reference-count": 21,
"references-count": 21,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-024-10216-3"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "The impact of dexamethasone on short- and long-term mortality in hospitalized COVID-19 patients: a retrospective study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "24"
}