Antisense therapy to block the Kallikrein-kinin pathway in COVID-19: The ASKCOV randomized controlled trial
et al., Journal of Critical Care, doi:10.1016/j.jcrc.2024.154892, ASKCOV, NCT04549922, Aug 2024
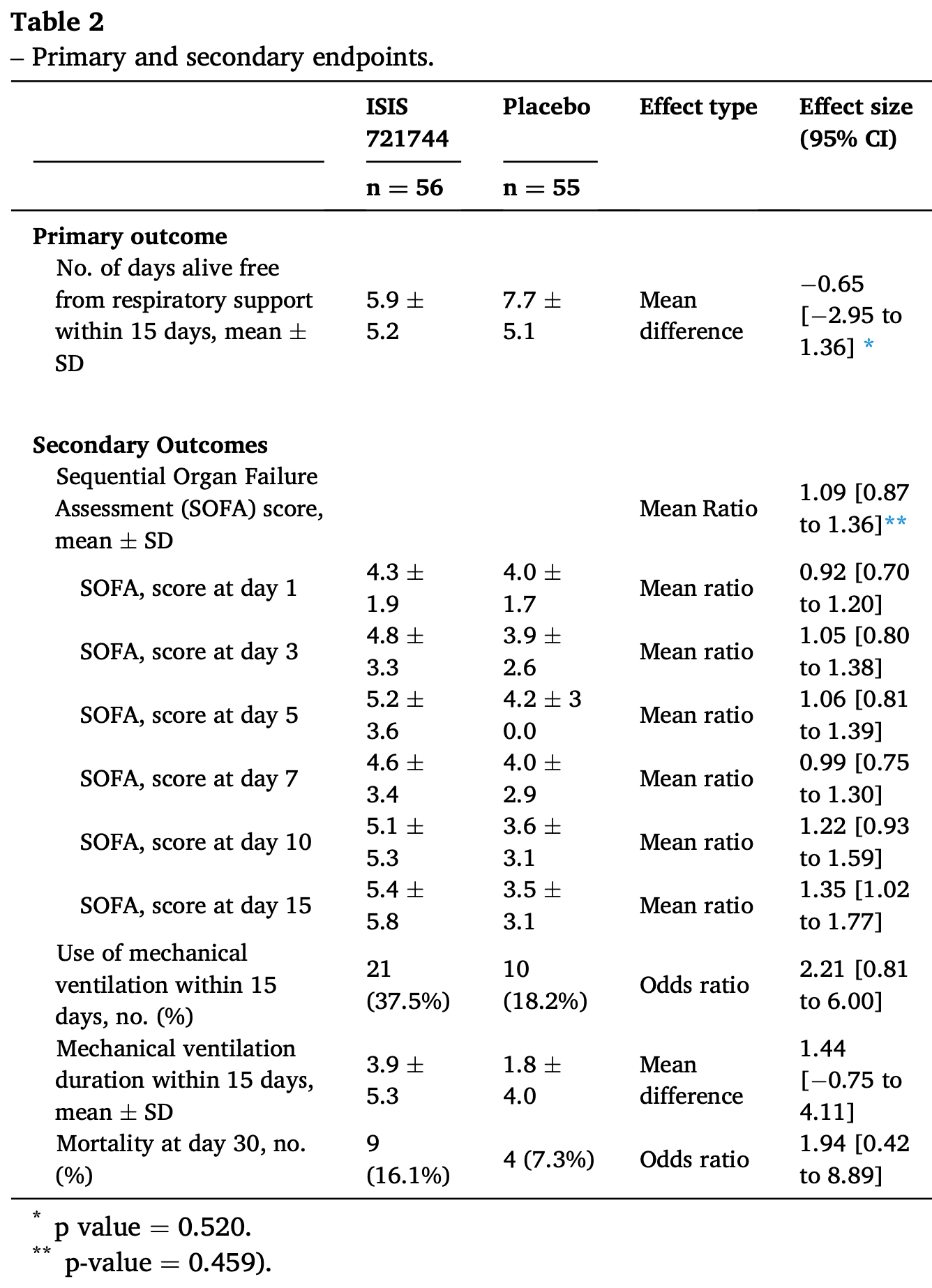
RCT 111 hospitalized COVID-19 patients in Brazil showing no clinical benefit with antisense therapy to block the kallikrein-kinin pathway using ISIS 721744 (donidalorsen). Treatment was associated with a significantly higher SOFA score at day 15 and with a significantly higher risk of adverse events.
|
risk of death, 151.0% higher, HR 2.51, p = 0.08, treatment 12 of 52 (23.1%), control 6 of 51 (11.8%), Cox proportional hazards, day 365.
|
|
risk of death, 81.6% higher, RR 1.82, p = 0.37, treatment 9 of 56 (16.1%), control 4 of 55 (7.3%), adjusted per study, odds ratio converted to relative risk, day 30.
|
|
risk of mechanical ventilation, 81.1% higher, RR 1.81, p = 0.08, treatment 21 of 56 (37.5%), control 10 of 55 (18.2%), adjusted per study, odds ratio converted to relative risk.
|
|
SOFA, 35.0% higher, OR 1.35, p = 0.03, treatment 56, control 55, adjusted per study, day 15, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Zampieri et al., 3 Aug 2024, Double Blind Randomized Controlled Trial, placebo-controlled, Brazil, peer-reviewed, mean age 57.5, 30 authors, study period 10 October, 2020 - 9 December, 2020, average treatment delay 7.9 days, trial NCT04549922 (history) (ASKCOV).
Contact: fzampier@ualberta.ca.
Antisense therapy to block the Kallikrein-kinin pathway in COVID-19: The ASKCOV randomized controlled trial
Journal of Critical Care, doi:10.1016/j.jcrc.2024.154892
Purpose: To assess the effect of antisense therapy to block kallikrein-kinin pathway in COVID-19 patients. Material and methods: Randomized, placebo-controlled, double blind, controlled trial enrolling hospitalized COVID-19 patients that required supplementary oxygen to sustain peripheral oxygen saturation. Key exclusion criteria included use of mechanical ventilation or vasopressors, and patients with more than 10 days since symptom onset or more than 48 h of oxygen use. Patients were randomized to either one subcutaneous dose of ISIS721744, an antisense that blocks prekallikrein, or placebo. The primary outcome was the number of days alive and free of oxygen support up to 15 days (DAFOR15). Secondary endpoints included organ failure score, need and duration of mechanical ventilation up to 15 days, and all-cause mortality at 30 days. Exploratory endpoints included physiological parameters, biomarkers, and quality of life. Results: From October 10, 2020, to December 09, 2020, 111 patients were randomized at thirteen sites in Brazil (56 to treatment and 55 to control group). Average age was 57.5 years, and most patients were male (68.5%). There were no significant differences in DAFOR15 between groups (5.9 ± 5.2 days for the intervention arm and 7.7 ± 5.1 for the control group; mean difference -0.65, 95% confidence intervals from -2.95 to 1.36, p = 0.520).
Declaration of competing interest None. This study was funded by Ionis Pharmaceutical, US, through a grant provided to HCor. The sponsor reviewed and agreed with the protocol, but had no role in any other aspect of the trial execution. MJ and GB were Ionis employees at the time this study was designed and provided relevant feedback on design of the trial and reviewed the final manuscript for intellectually relevant content. FGZ has received consulting fees from Baxter, unrelated to the scope of this manuscript.
Appendix A. Supplementary data Supplementary data to this article can be found online at https://doi. org/10.1016/j.jcrc.2024.154892.
References
Alexandre, Cavalcanti, Writingreview & editing, Writingoriginal draft
Cavalcanti, Zampieri, Rosa, Azevedo, Veiga et al., Coalition Covid-19 Brazil I Investigators. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19, N Engl J Med, doi:10.1056/NEJMoa2019014
Chauhan, Wiffen, Brown, COVID-19: A collision of complement, coagulation and inflammatory pathways, J Thromb Haemost, doi:10.1111/jth.14981
Cohn, Writingreview & editing, Visualization, Validation
Damiani, Writingoriginal draft
Fernando, Zampieri, Writingoriginal draft, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization
Fijen, Riedl, Bordone, Bernstein, Raasch et al., Inhibition of Prekallikrein for hereditary angioedema, N Engl J Med, doi:10.1056/NEJMoa2109329
Flávia, Machado, Writingreview & editing
Goligher, Lawler, Jensen, Talisa, Berry et al., Heterogeneous Treatment Effects of Therapeutic-Dose Heparin in Patients Hospitalized for COVID-19, JAMA, doi:10.1001/jama.2023.3651
Guimarães, Quirk, Furtado, Maia, Saraiva et al., STOP-COVID trial investigators. Tofacitinib in patients hospitalized with Covid-19 pneumonia, N Engl J Med, doi:10.1056/NEJMoa2101643
Horby, Lim, Emberson, Mafham, Bell et al., Dexamethasone in Hospitalized Patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Ishihara, Writingreview & editing, Visualization, Validation, Supervision, Methodology
Kalil, Patterson, Mehta, Tomashek, Wolfe et al., ACTT-2 Study Group Members. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2031994
Laranjeira, Writingreview & editing, Supervision, Funding acquisition, Conceptualization. Nanci Valeis: Writingreview & editing, Supervision, Project administration, Funding acquisition
Lipcsey, Persson, Eriksson, Blom, Fromell et al., The outcome of critically ill COVID-19 patients is linked to Thromboinflammation dominated by the Kallikrein/Kinin system, Front Immunol, doi:10.3389/fimmu.2021.627579.PMID:33692801;PMCID:PMC7937878
Luciano, Azevedo, Writingreview & editing, Investigation, Funding acquisition, Conceptualization
Martens, Van Mol, Wauters, Wauters, Gangnus et al., Dysregulation of the kallikrein-kinin system in bronchoalveolar lavage fluid of patients with severe COVID-19, EBioMedicine, doi:10.1016/j.ebiom.2022.104195
Mbuagbaw, Kosa, Lawson, Stalteri, Olaiya et al., The reporting of progression criteria in protocols of pilot trials designed to assess the feasibility of main trials is insufficient: a meta-epidemiological study, Pilot Feasibility Stud, doi:10.1186/s40814-019-0500-z.PMID:31700654;PMCID:PMC6827233
Negrelli, Writingreview & editing, Resources, Project administration, Funding acquisition
Polack, Thomas, Kitchin, Absalon, Gurtman et al., 4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine, N Engl J Med, doi:10.1056/NEJMoa2034577
Renato, Santos, Writingreview & editing, Visualization, Software, Project administration
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the new York City area, JAMA, doi:10.1001/jama.2020.6775
Roca, Caralt, Messika, Samper, Sztrymf et al., An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy, Am J Respir Crit Care Med, doi:10.1164/rccm.201803-0589OC
Samara, Gomes, Writingreview & editing, Project administration, Investigation, Data curation
Siemieniuk, Bartoszko, Zeraatkar, Kum, Qasim et al., Drug treatments for covid-19: living systematic review and network meta-analysis, BMJ, doi:10.1136/bmj.m2980
Skegg, Gluckman, Boulton, Hackmann, Karim et al., Future scenarios for the COVID-19 pandemic, Lancet, doi:10.1016/S0140-6736(21)00424-4
Souza, Writingreview & editing, Investigation, Data curation, Writingreview & editing, Investigation
Van De Veerdonk, Netea, Van Deuren, Van Der Meer, De Mast et al., Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome, Elife, doi:10.7554/eLife.57555.PMID:32338605;PMCID:PMC7213974
Vieira, Writingreview & editing, Project administration, Methodology, Funding acquisition
Vincent, Moreno, Takala, Willatts, Mendonça et al., The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on Sepsis-related problems of the European Society of Intensive Care Medicine, Intensive Care Med, doi:10.1007/BF01709751
Viviane, Veiga, Writingreview & editing, Investigation, Funding acquisition, Conceptualization
DOI record:
{
"DOI": "10.1016/j.jcrc.2024.154892",
"ISSN": [
"0883-9441"
],
"URL": "http://dx.doi.org/10.1016/j.jcrc.2024.154892",
"alternative-id": [
"S0883944124003794"
],
"article-number": "154892",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Antisense therapy to block the Kallikrein-kinin pathway in COVID-19: The ASKCOV randomized controlled trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Critical Care"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jcrc.2024.154892"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 The Authors. Published by Elsevier Inc."
}
],
"author": [
{
"affiliation": [],
"family": "Zampieri",
"given": "Fernando G.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Westphal",
"given": "Glauco Adrieno",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santos",
"given": "Maria Adelaide Dos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gomes",
"given": "Samara P.C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gomes",
"given": "Jackeline O.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Negrelli",
"given": "Karina L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santos",
"given": "Renato H.N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ishihara",
"given": "Luciana M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Miranda",
"given": "Tamiris A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Laranjeira",
"given": "Ligia N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Valeis",
"given": "Nanci",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santucci",
"given": "Eliana Vieira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Souza Dantas",
"given": "Vicente Cés",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gebara",
"given": "Otávio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cohn",
"given": "Danny M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Buchele",
"given": "Gustavo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Janiszewski",
"given": "Mariano",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Freitas",
"given": "Flávio Geraldo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dal-Pizzol",
"given": "Felipe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Matos Soeiro",
"given": "Alexandre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berti",
"given": "Isabele Ribeiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Germano",
"given": "Almir",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schettini",
"given": "Daniel Almeida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rosa",
"given": "Regis G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Falavigna",
"given": "Maicon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Veiga",
"given": "Viviane C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Azevedo",
"given": "Luciano C.P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Damiani",
"given": "Lucas P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Machado",
"given": "Flávia R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cavalcanti",
"given": "Alexandre B.",
"sequence": "additional"
}
],
"container-title": "Journal of Critical Care",
"container-title-short": "Journal of Critical Care",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
8,
2
]
],
"date-time": "2024-08-02T21:07:35Z",
"timestamp": 1722632855000
},
"deposited": {
"date-parts": [
[
2024,
8,
5
]
],
"date-time": "2024-08-05T19:02:33Z",
"timestamp": 1722884553000
},
"funder": [
{
"DOI": "10.13039/100013669",
"doi-asserted-by": "publisher",
"name": "Ionis Pharmaceuticals Inc"
}
],
"indexed": {
"date-parts": [
[
2024,
8,
6
]
],
"date-time": "2024-08-06T00:21:26Z",
"timestamp": 1722903686633
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
12
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
12,
1
]
],
"date-time": "2024-12-01T00:00:00Z",
"timestamp": 1733011200000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
12,
1
]
],
"date-time": "2024-12-01T00:00:00Z",
"timestamp": 1733011200000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
8,
5
]
],
"date-time": "2024-08-05T00:00:00Z",
"timestamp": 1722816000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0883944124003794?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0883944124003794?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "154892",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
12
]
]
},
"published-print": {
"date-parts": [
[
2024,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1001/jama.2020.6775",
"article-title": "Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the new York City area",
"author": "Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium; Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP",
"doi-asserted-by": "crossref",
"first-page": "2052",
"issue": "20",
"journal-title": "JAMA",
"key": "10.1016/j.jcrc.2024.154892_bb0005",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(21)00424-4",
"article-title": "Future scenarios for the COVID-19 pandemic",
"author": "Skegg",
"doi-asserted-by": "crossref",
"first-page": "777",
"issue": "10276",
"journal-title": "Lancet",
"key": "10.1016/j.jcrc.2024.154892_bb0010",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in Hospitalized Patients with Covid-19",
"author": "Horby",
"doi-asserted-by": "crossref",
"first-page": "693",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jcrc.2024.154892_bb0015",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2031994",
"article-title": "Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19",
"author": "Kalil",
"doi-asserted-by": "crossref",
"first-page": "795",
"issue": "9",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jcrc.2024.154892_bb0020",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2101643",
"article-title": "STOP-COVID trial investigators. Tofacitinib in patients hospitalized with Covid-19 pneumonia",
"author": "Guimarães",
"doi-asserted-by": "crossref",
"first-page": "406",
"issue": "5",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jcrc.2024.154892_bb0025",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m2980",
"article-title": "Drug treatments for covid-19: living systematic review and network meta-analysis",
"author": "Siemieniuk",
"doi-asserted-by": "crossref",
"first-page": "m2980",
"journal-title": "BMJ",
"key": "10.1016/j.jcrc.2024.154892_bb0030",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.7554/eLife.57555",
"article-title": "Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome",
"author": "van de Veerdonk",
"doi-asserted-by": "crossref",
"first-page": "e57555",
"issue": "9",
"journal-title": "Elife",
"key": "10.1016/j.jcrc.2024.154892_bb0035",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1016/j.ebiom.2022.104195",
"article-title": "Dysregulation of the kallikrein-kinin system in bronchoalveolar lavage fluid of patients with severe COVID-19",
"author": "Martens",
"doi-asserted-by": "crossref",
"first-page": "104195",
"journal-title": "EBioMedicine",
"key": "10.1016/j.jcrc.2024.154892_bb0040",
"volume": "83",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2021.627579",
"article-title": "The outcome of critically ill COVID-19 patients is linked to Thromboinflammation dominated by the Kallikrein/Kinin system",
"author": "Lipcsey",
"doi-asserted-by": "crossref",
"first-page": "627579",
"issue": "12",
"journal-title": "Front Immunol",
"key": "10.1016/j.jcrc.2024.154892_bb0045",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2109329",
"article-title": "Inhibition of Prekallikrein for hereditary angioedema",
"author": "Fijen",
"doi-asserted-by": "crossref",
"first-page": "1026",
"issue": "11",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jcrc.2024.154892_bb0050",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1007/BF01709751",
"article-title": "The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on Sepsis-related problems of the European Society of Intensive Care Medicine",
"author": "Vincent",
"doi-asserted-by": "crossref",
"first-page": "707",
"issue": "7",
"journal-title": "Intensive Care Med",
"key": "10.1016/j.jcrc.2024.154892_bb0055",
"volume": "22",
"year": "1996"
},
{
"DOI": "10.1164/rccm.201803-0589OC",
"article-title": "An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy",
"author": "Roca",
"doi-asserted-by": "crossref",
"first-page": "1368",
"issue": "11",
"journal-title": "Am J Respir Crit Care Med",
"key": "10.1016/j.jcrc.2024.154892_bb0060",
"volume": "199",
"year": "2019"
},
{
"DOI": "10.1111/jth.14981",
"article-title": "COVID-19: A collision of complement, coagulation and inflammatory pathways",
"author": "Chauhan",
"doi-asserted-by": "crossref",
"first-page": "2110",
"issue": "9",
"journal-title": "J Thromb Haemost",
"key": "10.1016/j.jcrc.2024.154892_bb0065",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2019014",
"article-title": "Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19",
"author": "Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, Damiani LP, Marcadenti A, Kawano-Dourado L, Lisboa T, Junqueira DLM, de Barros E Silva PGM, Tramujas L, Abreu-Silva EO, Laranjeira LN, Soares AT, Echenique LS, Pereira AJ, Freitas FGR, Gebara OCE, Dantas VCS, Furtado RHM, Milan EP, Golin NA, Cardoso FF, Maia IS, Hoffmann Filho CR, Kormann APM, Amazonas RB, Bocchi de Oliveira MF, Serpa-Neto A, Falavigna M, Lopes RD, Machado FR, Berwanger O; Coalition Covid-19 Brazil I Investigators",
"doi-asserted-by": "crossref",
"first-page": "2041",
"issue": "21",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jcrc.2024.154892_bb0070",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2034577",
"article-title": "Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine",
"author": "Polack",
"doi-asserted-by": "crossref",
"first-page": "2603",
"issue": "27",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jcrc.2024.154892_bb0075",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1001/jama.2023.3651",
"article-title": "Heterogeneous Treatment Effects of Therapeutic-Dose Heparin in Patients Hospitalized for COVID-19",
"author": "Goligher",
"doi-asserted-by": "crossref",
"first-page": "1066",
"issue": "13",
"journal-title": "JAMA",
"key": "10.1016/j.jcrc.2024.154892_bb0080",
"volume": "329",
"year": "2023"
},
{
"DOI": "10.1186/s40814-019-0500-z",
"article-title": "The reporting of progression criteria in protocols of pilot trials designed to assess the feasibility of main trials is insufficient: a meta-epidemiological study",
"author": "Mbuagbaw",
"doi-asserted-by": "crossref",
"first-page": "120",
"issue": "5",
"journal-title": "Pilot Feasibility Stud",
"key": "10.1016/j.jcrc.2024.154892_bb0085",
"volume": "3",
"year": "2019"
}
],
"reference-count": 17,
"references-count": 17,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0883944124003794"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"special_numbering": "C",
"subject": [],
"subtitle": [],
"title": "Antisense therapy to block the Kallikrein-kinin pathway in COVID-19: The ASKCOV randomized controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "84"
}