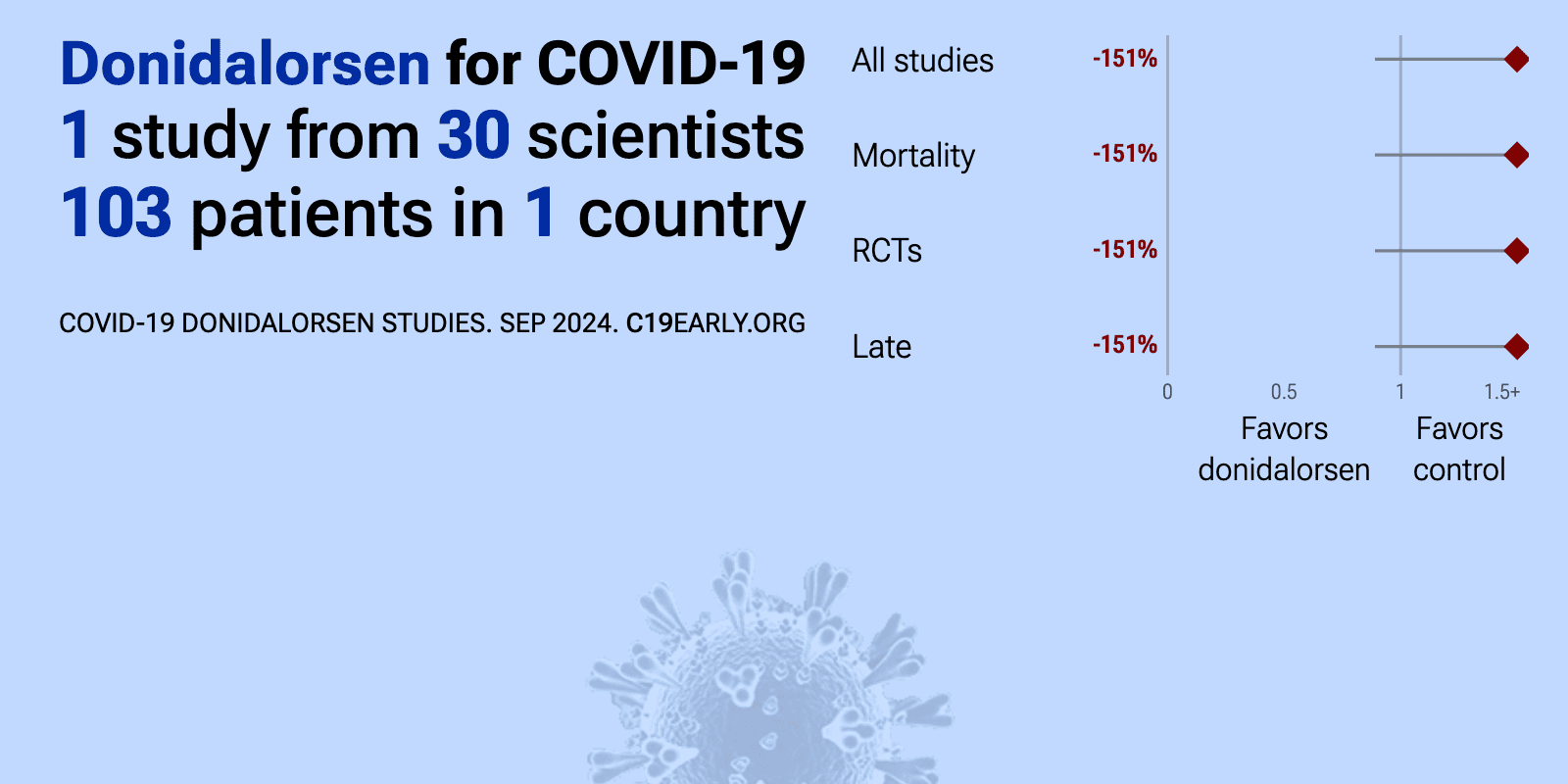
Aug 3 2024 |
et al., Journal of Critical Care, doi:10.1016/j.jcrc.2024.154892 | Antisense therapy to block the Kallikrein-kinin pathway in COVID-19: The ASKCOV randomized controlled trial |
| 151% higher mortality (p=0.08), 81% higher ventilation (p=0.08), and 35% higher progression (p=0.03). RCT 111 hospitalized COVID-19 patients in Brazil showing no clinical benefit with antisense therapy to block the kallikrein-kinin pathway using ISIS 721744 (donidalorsen). Treatment was associated with a significantly higher SOFA score at.. | ||