Hypertonic saline nasal irrigation and gargling for suspected or confirmed COVID-19: Pragmatic randomised controlled trial (ELVIS COVID-19)
et al., Journal of Global Health, doi:10.7189/jogh.14.05027, ELVIS COVID-19, NCT05104372, Dec 2024
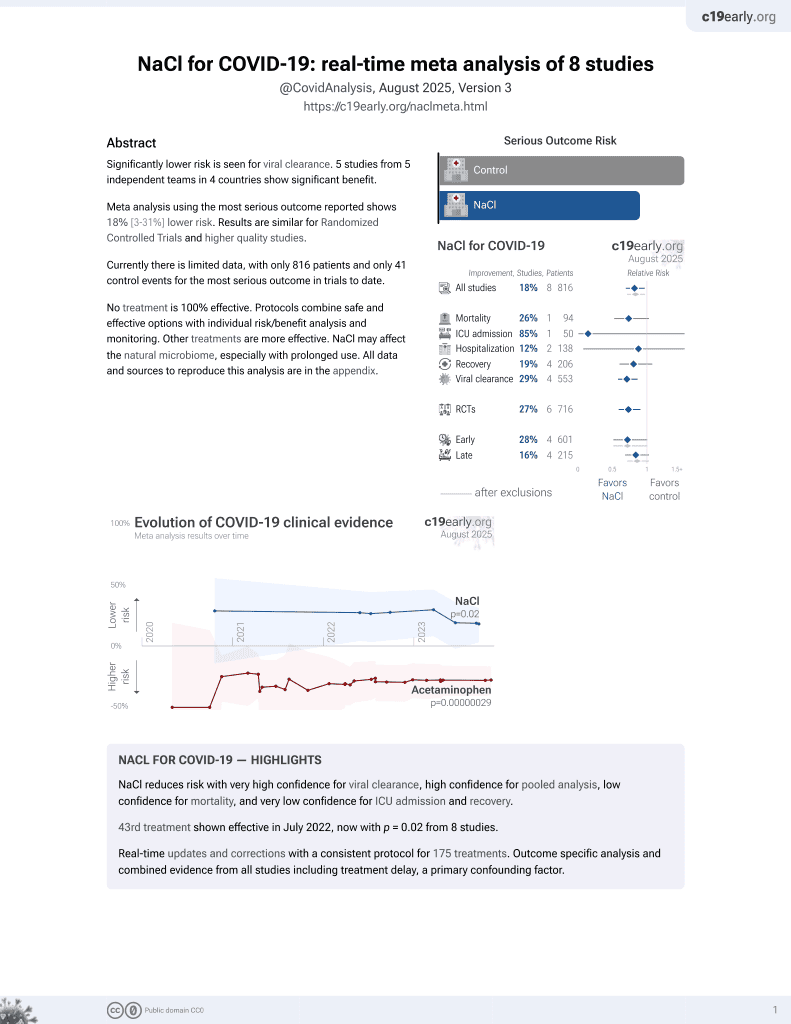
NaCl for COVID-19
44th treatment shown to reduce risk in
July 2022, now with p = 0.0028 from 9 studies.
Lower risk for progression and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 576 adults in Pakistan with suspected or confirmed COVID-19 showing no significant difference in time to symptom resolution with hypertonic saline nasal irrigation and gargling vs. nasal washing and gargling with tap water. Authors recommend testing earlier treatment.
Authors note that there were 78 patients with a clinician/laboratory confirmed positive COVID-19 PCR test and there was a notable imbalance in the distribution between arms (24 saline and 54 water) which they were unable to explain.
|
risk of no recovery, 18.7% lower, RR 0.81, p = 0.66, treatment 279, control 297, inverted to make RR<1 favor treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Yusuf et al., 13 Dec 2024, Randomized Controlled Trial, Pakistan, peer-reviewed, 11 authors, study period 1 May, 2021 - 15 October, 2021, this trial compares with another treatment - results may be better when compared to placebo, trial NCT05104372 (history) (ELVIS COVID-19).
Contact: aziz.sheikh@ed.ac.uk.
Hypertonic saline nasal irrigation and gargling for suspected or confirmed COVID-19: Pragmatic randomised controlled trial (ELVIS COVID-19)
Journal of Global Health, doi:10.7189/jogh.14.05027
Background In a previous pilot randomised controlled trial conducted on UK adults, we found that hypertonic saline nasal irrigation and gargling (HSNIG) reduced common cold symptoms, the need for over-the-counter medications, viral shedding, and the duration and transmission of the illness. It is unclear whether HSNIG improves outcomes of the coronavirus disease 2019 . Hypertonic saline can be prepared and HSNIG performed at home, making it a safe and scalable intervention, particularly well-suited for low-and middle-income countries.
Methods We conducted a pragmatic randomised controlled trial in Pakistan on adults with suspected or confirmed COVID-19, initially within 48 hours of symptom onset, later extended to within five days due to recruitment challenges. Participants were randomised to one of two groups: the intervention group received instructions on preparing a 2.6% hypertonic saline solution for HSNIG, while the control group was instructed on performing ablution for Muslim prayers (wudu), which involves nasal washing and gargling with tap water. Our primary outcome was the time to symptom resolution, measured by two consecutive days of scoring zero on relevant questions from the validated, self-reported, adapted short form of the Wisconsin Upper Respiratory Symptom Survey (WURSS-24). Secondary outcomes included the severity of all symptoms, the severity and time to resolution of individual symptoms, health care contacts (GP/physician, emergency contacts), hospital attendance (and length of stay if admitted), over-the-counter (OTC) medication (frequency and cost), and transmission to household contacts. The analysis was conducted on an intention-to-treat basis. Logistic regression was used to calculate adjusted odds ratios (aORs) of improvement and Cox regression to calculate adjusted hazard ratios (aHRs) for the time to improvement with accompanying 95% confidence intervals (CIs).
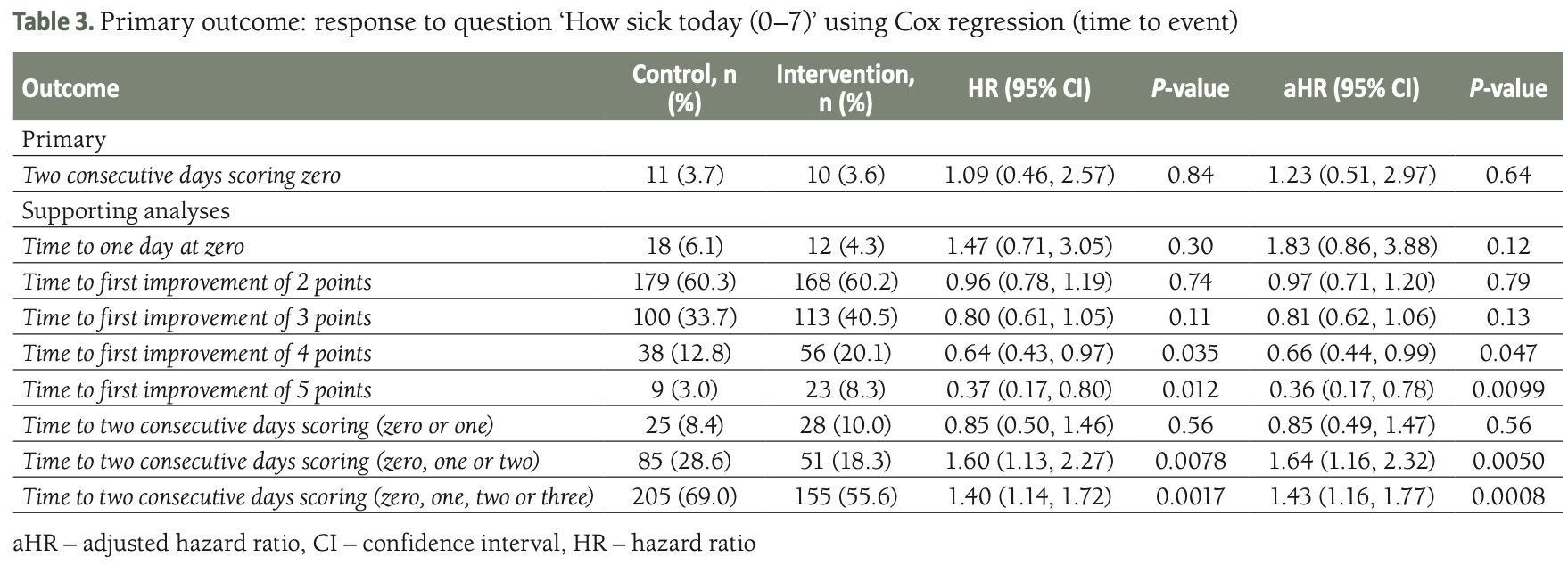
Results We randomised 576 people: 279 to the HSNIG group and 297 to the control group. Among those, 10 out of 279 (3.6%) in the HSNIG had symptom resolution, compared with 11 out of 297 (3.7%) in the control group (aOR = 1.20, 95% CI = 0.46-3.22). The time-to-event analysis also showed no significant benefit (aHR = 1.23, 95% CI = 0.51-2.97). Excluding the 127 participants with no data on the primary outcome (who did not complete the study), 10 out of 222 (4.5%) in the HSNIG group had symptom resolution, compared to 11 out of 227 (4.8%) in the control group.
References
Barrett, Brown, Mundt, Safdar, Dye et al., The Wisconsin Upper Respiratory Symptom Survey is responsive, reliable, and valid, J Clin Epidemiol, doi:10.1016/j.jclinepi.2004.11.019
Cabaillot, Vorilhon, Roca, Boussageon, Eschalier et al., Saline nasal irrigation for acute upper respiratory tract infections in infants and children: A systematic review and meta-analysis, Paediatr Respir Rev, doi:10.1016/j.prrv.2019.11.003
Esther Cr, Kimura, Mikami, Edwards, Das et al., Pharmacokinetic-based failure of a detergent virucidal for severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) nasal infections: A preclinical study and randomized controlled trial, Int Forum Allergy Rhinol, doi:10.1002/alr.22975
Gallo, Locatello, Mazzoni, Novelli, Annunziato, The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection, Mucosal Immunol, doi:10.1038/s41385-020-00359-2
House, Gadomski, Ralston, Evaluating the Placebo Status of Nebulized Normal Saline in Patients With Acute Viral Bronchiolitis: A Systematic Review and Meta-analysis, JAMA Pediatr, doi:10.1001/jamapediatrics.2019.5195
Kanjanawasee, Seresirikachorn, Chitsuthipakorn, Snidvongs, Hypertonic Saline Versus Isotonic Saline Nasal Irrigation: Systematic Review and Meta-analysis, Am J Rhinol Allergy, doi:10.1177/1945892418773566
Khan, Taj, Bano, Ullah, Khan, Occurrence of Chlorine Resistant Bacteria in Drinking Water Filtration Plants of Rawalpindi City, Pakistan, Frontiers in Environmental Microbiology, doi:10.11648/j.fem.20210701.12
King, What role for saline nasal irrigation?, Drug Ther Bull, doi:10.1136/dtb.2018.000023
Rabago, Zgierska, Saline nasal irrigation for upper respiratory conditions, Am Fam Physician
Ramalingam, Cai, Wong, Twomey, Chen et al., Antiviral innate immune response in non-myeloid cells is augmented by chloride ions via an increase in intracellular hypochlorous acid levels, Sci Rep, doi:10.1038/s41598-018-31936-y
Ramalingam, Graham, Dove, Morrice, Sheikh, A pilot, open labelled, randomised controlled trial of hypertonic saline nasal irrigation and gargling for the common cold, Sci Rep, doi:10.1038/s41598-018-37703-3
Ramalingam, Graham, Dove, Morrice, Sheikh, Hypertonic saline nasal irrigation and gargling should be considered as a treatment option for COVID-19, J Glob Health, doi:10.7189/jogh.10.010332
Signorini, Leung, Simes, Beller, Gebski et al., Dynamic balanced randomization for clinical trials, Stat Med, doi:10.1002/sim.4780122410
Slapak, Skoupá, Strnad, Horník, Efficacy of isotonic nasal wash (seawater) in the treatment and prevention of rhinitis in children, Arch Otolaryngol Head Neck Surg, doi:10.1001/archoto.2007.19
DOI record:
{
"DOI": "10.7189/jogh.14.05027",
"ISSN": [
"2047-2978",
"2047-2986"
],
"URL": "http://dx.doi.org/10.7189/jogh.14.05027",
"article-number": "05027",
"author": [
{
"affiliation": [
{
"name": "The Allergy & Asthma Institute, Islamabad, Pakistan"
}
],
"family": "Yusuf",
"given": "Osman M",
"sequence": "first"
},
{
"affiliation": [
{
"name": "National Health Service (NHS) Lothian, Edinburgh, UK"
}
],
"family": "Ramalingam",
"given": "Sandeep",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Edinburgh Clinical Trials Unit, Usher Institute, University of Edinburgh, Edinburgh, UK"
}
],
"family": "Norrie",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Edinburgh Clinical Research Facility, University of Edinburgh, Edinburgh, UK"
}
],
"family": "Graham",
"given": "Catriona",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The Allergy & Asthma Institute, Islamabad, Pakistan"
},
{
"name": "National University of Modern Languages, Islamabad"
}
],
"family": "Kakakhail",
"given": "Ahmad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The Allergy & Asthma Institute, Islamabad, Pakistan"
},
{
"name": "National University of Sciences and Technology (NUST), Islamabad"
}
],
"family": "Rextin",
"given": "Aimal T",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The Allergy & Asthma Institute, Islamabad, Pakistan"
}
],
"family": "Baig",
"given": "Ramsha T",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The Allergy & Asthma Institute, Islamabad, Pakistan"
}
],
"family": "Yusuf",
"given": "Shahida O",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The Allergy & Asthma Institute, Islamabad, Pakistan"
}
],
"family": "Ahmad",
"given": "Bakhtawar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The Allergy & Asthma Institute, Islamabad, Pakistan"
}
],
"family": "Zahra",
"given": "Summan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Usher Institute, University of Edinburgh, Edinburgh, UK"
},
{
"name": "Nuffield Department of Primary Care Health Sciences, University of Oxford, Oxford, UK"
}
],
"family": "Sheikh",
"given": "Aziz",
"sequence": "additional"
}
],
"container-title": "Journal of Global Health",
"container-title-short": "J Glob Health",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
12,
12
]
],
"date-time": "2024-12-12T17:56:36Z",
"timestamp": 1734026196000
},
"deposited": {
"date-parts": [
[
2024,
12,
12
]
],
"date-time": "2024-12-12T17:56:38Z",
"timestamp": 1734026198000
},
"indexed": {
"date-parts": [
[
2024,
12,
13
]
],
"date-time": "2024-12-13T05:28:55Z",
"timestamp": 1734067735618,
"version": "3.30.2"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
12,
13
]
]
},
"link": [
{
"URL": "https://jogh.org/2024/jogh-14-05027",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "4223",
"original-title": [],
"prefix": "10.7189",
"published": {
"date-parts": [
[
2024,
12,
13
]
]
},
"published-online": {
"date-parts": [
[
2024,
12,
13
]
]
},
"publisher": "International Society of Global Health",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"article-title": "A Novel Coronavirus from Patients with Pneumonia in China, 2019.",
"author": "Zhu",
"doi-asserted-by": "publisher",
"first-page": "727",
"journal-title": "N Engl J Med",
"key": "key-10.7189/jogh.14.05027-202412121443-R1",
"volume": "382",
"year": "2020"
},
{
"key": "key-10.7189/jogh.14.05027-202412121443-R2",
"unstructured": "Worldometer. Coronavirus. 2024. Available: https://www.worldometers.info/coronavirus/. Accessed: 4 January 2024."
},
{
"DOI": "10.1038/s41564-020-0695-z",
"article-title": "The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2.",
"author": "Coronaviridae Study Group of the International Committee on Taxonomy of Viruses",
"doi-asserted-by": "publisher",
"first-page": "536",
"journal-title": "Nat Microbiol",
"key": "key-10.7189/jogh.14.05027-202412121443-R3",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1002/jmv.28103",
"article-title": "Stability and transmissibility of SARS-CoV-2 in the environment.",
"author": "Geng",
"doi-asserted-by": "publisher",
"first-page": "e28103",
"journal-title": "J Med Virol",
"key": "key-10.7189/jogh.14.05027-202412121443-R4",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(21)01358-1",
"article-title": "Public Health Scotland and the EAVE II Collaborators. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness.",
"author": "Sheikh",
"doi-asserted-by": "publisher",
"first-page": "2461",
"journal-title": "Lancet",
"key": "key-10.7189/jogh.14.05027-202412121443-R5",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1016/S1473-3099(22)00451-0",
"article-title": "Vaccine effectiveness of two-dose BNT162b2 against symptomatic and severe COVID-19 among adolescents in Brazil and Scotland over time: a test-negative case-control study.",
"author": "Florentino",
"doi-asserted-by": "publisher",
"first-page": "1577",
"journal-title": "Lancet Infect Dis",
"key": "key-10.7189/jogh.14.05027-202412121443-R6",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(22)00141-4",
"article-title": "EAVE II Collaborators. Severity of omicron variant of concern and effectiveness of vaccine boosters against symptomatic disease in Scotland (EAVE II): a national cohort study with nested test-negative design.",
"author": "Sheikh",
"doi-asserted-by": "publisher",
"first-page": "959",
"journal-title": "Lancet Infect Dis",
"key": "key-10.7189/jogh.14.05027-202412121443-R7",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)01656-7",
"article-title": "Severe COVID-19 outcomes after full vaccination of primary schedule and initial boosters: pooled analysis of national prospective cohort studies of 30 million individuals in England, Northern Ireland, Scotland, and Wales.",
"author": "Agrawal",
"doi-asserted-by": "publisher",
"first-page": "1305",
"journal-title": "Lancet",
"key": "key-10.7189/jogh.14.05027-202412121443-R8",
"volume": "400",
"year": "2022"
},
{
"DOI": "10.1038/s41385-020-00359-2",
"article-title": "The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection.",
"author": "Gallo",
"doi-asserted-by": "publisher",
"first-page": "305",
"journal-title": "Mucosal Immunol",
"key": "key-10.7189/jogh.14.05027-202412121443-R9",
"volume": "14",
"year": "2021"
},
{
"article-title": "Saline nasal irrigation for upper respiratory conditions.",
"author": "Rabago",
"first-page": "1117",
"journal-title": "Am Fam Physician",
"key": "key-10.7189/jogh.14.05027-202412121443-R10",
"volume": "80",
"year": "2009"
},
{
"DOI": "10.1136/dtb.2018.000023",
"article-title": "What role for saline nasal irrigation?",
"author": "King",
"doi-asserted-by": "publisher",
"first-page": "56",
"journal-title": "Drug Ther Bull",
"key": "key-10.7189/jogh.14.05027-202412121443-R11",
"volume": "57",
"year": "2019"
},
{
"DOI": "10.1177/1945892418773566",
"article-title": "Hypertonic Saline Versus Isotonic Saline Nasal Irrigation: Systematic Review and Meta-analysis.",
"author": "Kanjanawasee",
"doi-asserted-by": "publisher",
"first-page": "269",
"journal-title": "Am J Rhinol Allergy",
"key": "key-10.7189/jogh.14.05027-202412121443-R12",
"volume": "32",
"year": "2018"
},
{
"DOI": "10.1038/s41598-018-37703-3",
"article-title": "A pilot, open labelled, randomised controlled trial of hypertonic saline nasal irrigation and gargling for the common cold.",
"author": "Ramalingam",
"doi-asserted-by": "publisher",
"first-page": "1015",
"journal-title": "Sci Rep",
"key": "key-10.7189/jogh.14.05027-202412121443-R13",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1016/j.prrv.2019.11.003",
"article-title": "Saline nasal irrigation for acute upper respiratory tract infections in infants and children: A systematic review and meta-analysis.",
"author": "Cabaillot",
"doi-asserted-by": "publisher",
"first-page": "151",
"journal-title": "Paediatr Respir Rev",
"key": "key-10.7189/jogh.14.05027-202412121443-R14",
"volume": "36",
"year": "2020"
},
{
"DOI": "10.1001/archoto.2007.19",
"article-title": "Efficacy of isotonic nasal wash (seawater) in the treatment and prevention of rhinitis in children.",
"author": "Slapak",
"doi-asserted-by": "publisher",
"first-page": "67",
"journal-title": "Arch Otolaryngol Head Neck Surg",
"key": "key-10.7189/jogh.14.05027-202412121443-R15",
"volume": "134",
"year": "2008"
},
{
"DOI": "10.1038/s41598-018-31936-y",
"article-title": "Antiviral innate immune response in non-myeloid cells is augmented by chloride ions via an increase in intracellular hypochlorous acid levels.",
"author": "Ramalingam",
"doi-asserted-by": "publisher",
"first-page": "13630",
"journal-title": "Sci Rep",
"key": "key-10.7189/jogh.14.05027-202412121443-R16",
"volume": "8",
"year": "2018"
},
{
"DOI": "10.7189/jogh.10.010332",
"article-title": "Hypertonic saline nasal irrigation and gargling should be considered as a treatment option for COVID-19.",
"author": "Ramalingam",
"doi-asserted-by": "publisher",
"first-page": "010332",
"journal-title": "J Glob Health",
"key": "key-10.7189/jogh.14.05027-202412121443-R17",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1002/sim.4780122410",
"article-title": "Dynamic balanced randomization for clinical trials.",
"author": "Signorini",
"doi-asserted-by": "publisher",
"first-page": "2343",
"journal-title": "Stat Med",
"key": "key-10.7189/jogh.14.05027-202412121443-R18",
"volume": "12",
"year": "1993"
},
{
"DOI": "10.1016/j.jclinepi.2004.11.019",
"article-title": "The Wisconsin Upper Respiratory Symptom Survey is responsive, reliable, and valid.",
"author": "Barrett",
"doi-asserted-by": "publisher",
"first-page": "609",
"journal-title": "J Clin Epidemiol",
"key": "key-10.7189/jogh.14.05027-202412121443-R19",
"volume": "58",
"year": "2005"
},
{
"DOI": "10.11648/j.fem.20210701.12",
"article-title": "Occurrence of Chlorine Resistant Bacteria in Drinking Water Filtration Plants of Rawalpindi City, Pakistan.",
"author": "Khan",
"doi-asserted-by": "publisher",
"first-page": "6",
"journal-title": "Frontiers in Environmental Microbiology",
"key": "key-10.7189/jogh.14.05027-202412121443-R20",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1001/jamapediatrics.2019.5195",
"article-title": "Evaluating the Placebo Status of Nebulized Normal Saline in Patients With Acute Viral Bronchiolitis: A Systematic Review and Meta-analysis.",
"author": "House",
"doi-asserted-by": "publisher",
"first-page": "250",
"journal-title": "JAMA Pediatr",
"key": "key-10.7189/jogh.14.05027-202412121443-R21",
"volume": "174",
"year": "2020"
},
{
"DOI": "10.1002/alr.22975",
"article-title": "Pharmacokinetic-based failure of a detergent virucidal for severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) nasal infections: A preclinical study and randomized controlled trial.",
"author": "Esther",
"doi-asserted-by": "publisher",
"first-page": "1137",
"journal-title": "Int Forum Allergy Rhinol",
"key": "key-10.7189/jogh.14.05027-202412121443-R22",
"volume": "12",
"year": "2022"
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "https://jogh.org/2024/jogh-14-05027"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Hypertonic saline nasal irrigation and gargling for suspected or confirmed COVID-19: Pragmatic randomised controlled trial (ELVIS COVID-19)",
"type": "journal-article",
"volume": "14"
}