Interferon-α Nasal Spray Prophylaxis Reduces COVID-19 in Cancer Patients: A Randomized, Double-Blinded, Placebo-Controlled Trial
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciaf409, NCT04534725, Aug 2025
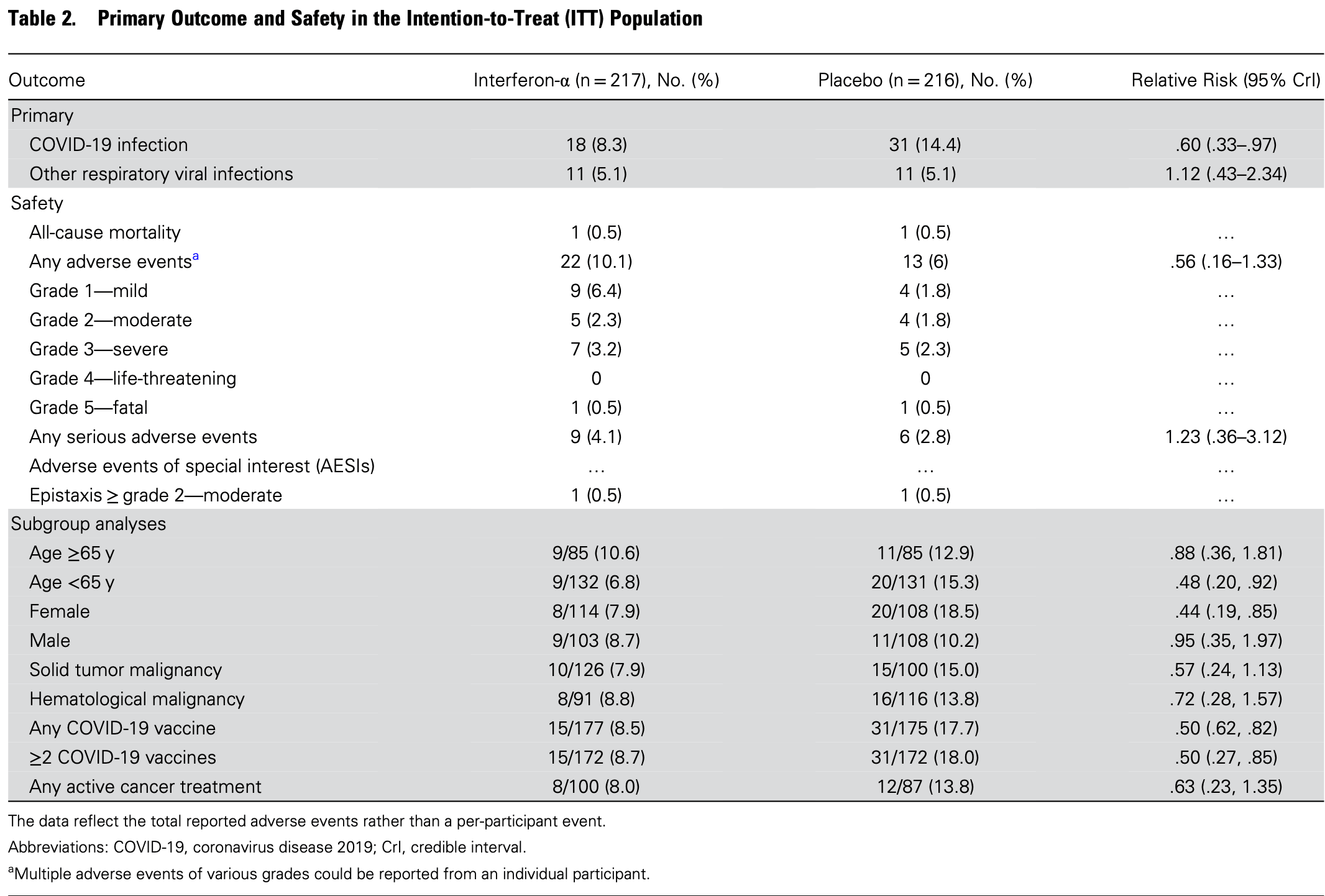
RCT 433 adult cancer patients showing lower risk of COVID-19 infection with daily interferon-alpha nasal spray prophylaxis compared to placebo over 90 days. There was no significant difference for hospitalization. Authors hypothesize that interferon-alpha's broad antiviral and immunomodulatory effects, particularly its role in innate immunity against respiratory viruses, explain the protective effect against COVID-19. Data is unclear - the count and percentage is inconsistent for infection-related hospitalization in the placebo group (authors report 7/216 (3.7%) and repeat 3.7% in the main text).
|
risk of hospitalization, 13.8% higher, RR 1.14, p = 1.00, treatment 8 of 217 (3.7%), control 7 of 216 (3.2%).
|
|
risk of case, 42.2% lower, RR 0.58, p = 0.0498, treatment 18 of 217 (8.3%), control 31 of 216 (14.4%), NNT 17.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Yong et al., 28 Aug 2025, Double Blind Randomized Controlled Trial, placebo-controlled, Australia, peer-reviewed, 21 authors, trial NCT04534725 (history).
Contact: michelle.yong@unimelb.edu.au, michelle.yong@petermac.org.au.
Interferon-α Nasal Spray Prophylaxis Reduces COVID-19 in Cancer Patients: A Randomized, Double-Blinded, Placebo-Controlled Trial
doi:10.1093/cid/ciaf409/8241089
Background. We evaluated whether a daily nasal spray of interferon-alpha (IFN-α) would reduce the incidence of COVID-19 or community-acquired respiratory viral infections in adult cancer patients. Methods. In this multicenter, randomized, double-blinded, placebo-controlled trial, participants were randomized 1:1 to receive daily 40 000 IU IFN-α nasal spray or normal saline placebo. Participants who developed influenza-like symptoms selfcollected nasal swabs for PCR testing of SARS-CoV-2, influenza A/B, respiratory syncytial virus, parainfluenza, adenovirus, seasonal coronavirus, picornavirus, human metapneumovirus, and/or SARS-CoV-2 rapid antigen testing. Co-primary endpoints were incidence of COVID-19 and/or other respiratory viruses ≤90 days of randomization. Results. Four hundred and thirty-three participants were randomized to IFN-α (n = 217) or placebo (n = 216). The incidence of COVID-19 was lower in the IFN-α group versus placebo (8.3% vs 14.4%), indicating a 40% reduced risk of infection (relative risk [RR]: .60; 95% credible interval [CrI]: .33-.97). Other respiratory viral infection incidence was 5.1% and 5.1% in both groups (RR: 1.12; .43-2.34). In the per-protocol cohort (n = 389), the incidence of COVID-19 in IFN-α and placebo groups was 7.7% and 16.0% (RR: .50; .26-.84) with other respiratory virus incidence of 4.6% and 5.7%, respectively. Subgroup analysis demonstrated lower COVID-19 in the IFN-α group for ages <65 years (RR: .48; .20-.92), female sex (RR: .44;), but no difference by underlying malignancy. No differences were observed in secondary endpoints of severity, hospitalization, and mortality. IFN-α was well tolerated and safe. Conclusions. IFN-α nasal spray prophylaxis reduced the incidence of COVID-19 among adult cancer patients. Clinical Trials Registration. ClinicalTrials.
Supplementary Data Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes Acknowledgments. The authors thank all the study participants and their carers.
References
Boeckh, Pergam, Limaye, Englund, Corey et al., How immunocompromised hosts were left behind in the quest to control the COVID-19 pandemic, Clin Infect Dis
Calabrese, Winthrop, Strand, Yazdany, Walter, Type I interferon, anti-interferon antibodies, and COVID-19, Lancet Rheumatol
Douglas, Moore, Miles, Prophylactic efficacy of intranasal alpha 2-interferon against rhinovirus infections in the family setting, N Engl J Med
Farr, Gwaltney, Jr, Adams, Hayden, Intranasal interferon-alpha 2 for prevention of natural rhinovirus colds, Antimicrob Agents Chemother
Fillmore, La, Szalat, Prevalence and outcome of COVID-19 infection in cancer patients: a national Veterans Affairs study, J Natl Cancer Inst
Gao, Yu, Chen, A randomized controlled trial of low-dose recombinant human interferons alpha-2b nasal spray to prevent acute viral respiratory infections in military recruits, Vaccine
Hall, Lim, Saunders, Breakthrough COVID-19 is mild in vaccinated patients with hematological malignancy receiving tixagevimab-cilgavimab as pre-exposure prophylaxis, Leuk Lymphoma
Hall, Nguyen, Allen, Evolution of humoral and cellular immunity post-breakthrough coronavirus disease 2019 in vaccinated patients with hematologic malignancy receiving tixagevimab-cilgavimab, Open Forum Infect Dis
Hall, Sim, Lim, COVID-19 infection among patients with cancer in Australia from 2020 to 2022: a national multicentre cohort study, Lancet Reg Health West Pac
Hall, Smibert, Sullivan, Influenza vaccination strategies in patients with hematologic cancer, N Engl J Med
Hayden, Albrecht, Kaiser, Gwaltney, Prevention of natural colds by contact prophylaxis with intranasal alpha 2-interferon, N Engl J Med
Imanishi, Karaki, Sasaki, The preventive effect of human interferon-alpha preparation on upper respiratory disease, J Interferon Res
Johansson, Skog, Johannesen, Were cancer patients worse off than the general population during the COVID-19 pandemic? A populationbased study from Norway, Denmark and Iceland during the pre-vaccination era, Lancet Reg Health Europe
Luo, Connell, Yu, Cancer incidence and mortality in Australia from 2020 to 2044 and an exploratory analysis of the potential effect of treatment delays during the COVID-19 pandemic: a statistical modelling study, Lancet Public Health
Meng, Wang, Chen, The effect of recombinant human interferon alpha nasal drops to prevent COVID-19 pneumonia for medical staff in an epidemic area, Curr Top Med Chem
Pagano, Salmanton-García, Marchesi, COVID-19 infection in adult patients with hematological malignancies: a European Hematology Association Survey (EPICOVIDEHA), J Hematol Oncol
Reis, Silva, Silva, Early treatment with pegylated interferon lambda for Covid-19, N Engl J Med
Sallard, Lescure, Yazdanpanah, Mentre, Peiffer-Smadja, Type 1 interferons as a potential treatment against COVID-19, Antiviral Res
Teh, Coussement, Neoh, Immunogenicity of COVID-19 vaccines in patients with hematologic malignancies: a systematic review and meta-analysis, Blood Adv
Zhang, Bastard, Liu, Inborn errors of type I IFN immunity in patients with life-threatening COVID-19, Science
Zhou, Chen, Lu, Wang, Yu, Interferon-α-2b nasal spray for treating SARS-CoV-2 omicron variant-infected children, J Clin Immunol
DOI record:
{
"DOI": "10.1093/cid/ciaf409",
"ISSN": [
"1058-4838",
"1537-6591"
],
"URL": "http://dx.doi.org/10.1093/cid/ciaf409",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>We evaluated whether a daily nasal spray of interferon-alpha (IFN-α) would reduce the incidence of COVID-19 or community-acquired respiratory viral infections in adult cancer patients.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>In this multicenter, randomized, double-blinded, placebo-controlled trial, participants were randomized 1:1 to receive daily 40 000 IU IFN-α nasal spray or normal saline placebo. Participants who developed influenza-like symptoms self-collected nasal swabs for PCR testing of SARS-CoV-2, influenza A/B, respiratory syncytial virus, parainfluenza, adenovirus, seasonal coronavirus, picornavirus, human metapneumovirus, and/or SARS-CoV-2 rapid antigen testing. Co-primary endpoints were incidence of COVID-19 and/or other respiratory viruses ≤90 days of randomization.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Four hundred and thirty-three participants were randomized to IFN-α (n = 217) or placebo (n = 216). The incidence of COVID-19 was lower in the IFN-α group versus placebo (8.3% vs 14.4%), indicating a 40% reduced risk of infection (relative risk [RR]: .60; 95% credible interval [CrI]: .33–.97). Other respiratory viral infection incidence was 5.1% and 5.1% in both groups (RR: 1.12; .43–2.34). In the per-protocol cohort (n = 389), the incidence of COVID-19 in IFN-α and placebo groups was 7.7% and 16.0% (RR: .50; .26–.84) with other respiratory virus incidence of 4.6% and 5.7%, respectively. Subgroup analysis demonstrated lower COVID-19 in the IFN-α group for ages &lt;65 years (RR: .48; .20–.92), female sex (RR: .44; .19–.85), and COVID-19 vaccinated (RR: .50; .26–.82), but no difference by underlying malignancy. No differences were observed in secondary endpoints of severity, hospitalization, and mortality. IFN-α was well tolerated and safe.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>IFN-α nasal spray prophylaxis reduced the incidence of COVID-19 among adult cancer patients.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Clinical Trials Registration</jats:title>\n <jats:p>ClinicalTrials.gov identifier: NCT04534725 (ANZCTR: ACTRN12620000843954)</jats:p>\n </jats:sec>",
"article-number": "ciaf409",
"author": [
{
"ORCID": "https://orcid.org/0000-0002-8692-4145",
"affiliation": [
{
"name": "Department of Infectious Diseases, Peter MacCallum Cancer Centre , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "Sir Peter MacCallum Department of Oncology, The University of Melbourne , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "Department of Infectious Diseases, Royal Melbourne Hospital , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "National Centre for Infections in Cancer, Peter MacCallum Cancer Centre , Melbourne, Victoria ,",
"place": [
"Australia"
]
}
],
"authenticated-orcid": false,
"family": "Yong",
"given": "Michelle K",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-7400-232X",
"affiliation": [
{
"name": "Department of Infectious Diseases, Peter MacCallum Cancer Centre , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "Sir Peter MacCallum Department of Oncology, The University of Melbourne , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "National Centre for Infections in Cancer, Peter MacCallum Cancer Centre , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "Department of Health Services Research, Peter MacCallum Cancer Centre , Melbourne, Victoria ,",
"place": [
"Australia"
]
}
],
"authenticated-orcid": false,
"family": "Thursky",
"given": "Karin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Peter MacCallum Cancer Centre , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "National Centre for Infections in Cancer, Peter MacCallum Cancer Centre , Melbourne, Victoria ,",
"place": [
"Australia"
]
}
],
"family": "Crane",
"given": "Megan",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-9204-3216",
"affiliation": [
{
"name": "Sir Peter MacCallum Department of Oncology, The University of Melbourne , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "National Centre for Infections in Cancer, Peter MacCallum Cancer Centre , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "Department of Health Services Research, Peter MacCallum Cancer Centre , Melbourne, Victoria ,",
"place": [
"Australia"
]
}
],
"authenticated-orcid": false,
"family": "Spelman",
"given": "Tim",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-3643-923X",
"affiliation": [
{
"name": "Biostatistics Unit, Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, University of Melbourne , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "Methods and Implementation Support for Clinical and Health (MISCH) Research Hub, The University of Melbourne , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "Clinical Epidemiology and Biostatistics Unit, Murdoch Children's Research Institute , Parkville, Victoria ,",
"place": [
"Australia"
]
}
],
"authenticated-orcid": false,
"family": "Mahar",
"given": "Robert K",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-2660-2013",
"affiliation": [
{
"name": "Biostatistics Unit, Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, University of Melbourne , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "Methods and Implementation Support for Clinical and Health (MISCH) Research Hub, The University of Melbourne , Melbourne, Victoria ,",
"place": [
"Australia"
]
}
],
"authenticated-orcid": false,
"family": "Simpson",
"given": "Julie A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Molecular Imaging and Therapy, Austin Health , Heidelberg, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "Olivia Newton-John Cancer Research Institute , Tumour Targeting Laboratory, Heidelberg, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "Faculty of Medicine, University of Melbourne , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "School of Cancer Medicine, LaTrobe University , Melbourne, Victoria ,",
"place": [
"Australia"
]
}
],
"family": "Scott",
"given": "Andrew M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Sir Peter MacCallum Department of Oncology, The University of Melbourne , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "National Centre for Infections in Cancer, Peter MacCallum Cancer Centre , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "Clinical Haematology, Peter MacCallum Cancer Centre and Royal Melbourne Hospital , Melbourne, Victoria ,",
"place": [
"Australia"
]
}
],
"family": "Harrison",
"given": "Simon J",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-6783-2301",
"affiliation": [
{
"name": "Faculty of Medicine, University of Melbourne , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "Clinical Haematology, Peter MacCallum Cancer Centre and Royal Melbourne Hospital , Melbourne, Victoria ,",
"place": [
"Australia"
]
}
],
"authenticated-orcid": false,
"family": "Szer",
"given": "Jeff",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-3627-3126",
"affiliation": [
{
"name": "Walter and Eliza Hall Institute of Medical Research , Division of Infectious Diseases and Immune Defence, Parkville, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "Department of Medical Biology, University of Melbourne , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "Centenary Institute, Executive Director , Camperdown, New South Wales ,",
"place": [
"Australia"
]
},
{
"name": "Faculty of Heath and Medical Sciences, University of Sydney , Camperdown, New South Wales ,",
"place": [
"Australia"
]
}
],
"authenticated-orcid": false,
"family": "Pellegrini",
"given": "Marc",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-2678-5208",
"affiliation": [
{
"name": "Sir Peter MacCallum Department of Oncology, The University of Melbourne , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "Pharmacy Department, Peter MacCallum Cancer Centre , Melbourne, Victoria ,",
"place": [
"Australia"
]
}
],
"authenticated-orcid": false,
"family": "Lingaratnam",
"given": "Senthil",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-6881-775X",
"affiliation": [
{
"name": "Clinical Sciences, Murdoch Children's Research Institute , Parkville, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "Department of Adolescent Medicine, Royal Children's Hospital , Parkville, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "Department of Paediatrics, University of Melbourne , Parkville, Victoria ,",
"place": [
"Australia"
]
}
],
"authenticated-orcid": false,
"family": "Pang",
"given": "Ken C",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-7919-7226",
"affiliation": [
{
"name": "Department of Infectious Diseases, Peter MacCallum Cancer Centre , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "National Centre for Infections in Cancer, Peter MacCallum Cancer Centre , Melbourne, Victoria ,",
"place": [
"Australia"
]
}
],
"authenticated-orcid": false,
"family": "Tennakoon",
"given": "Surekha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Sir Peter MacCallum Department of Oncology, The University of Melbourne , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "National Centre for Infections in Cancer, Peter MacCallum Cancer Centre , Melbourne, Victoria ,",
"place": [
"Australia"
]
}
],
"family": "Sim",
"given": "Beatrice Z",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-7849-7139",
"affiliation": [
{
"name": "Department of Haematology, Westmead Hospital , Westmead, New South Wales ,",
"place": [
"Australia"
]
},
{
"name": "Westmead Institute for Medical Research, University of Sydney , Sydney, New South Wales ,",
"place": [
"Australia"
]
}
],
"authenticated-orcid": false,
"family": "Blyth",
"given": "Emily",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-7319-8546",
"affiliation": [
{
"name": "Department of Oncology, Austin Hospital , Heidelberg, Victoria ,",
"place": [
"Australia"
]
}
],
"authenticated-orcid": false,
"family": "Gan",
"given": "Hui K",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-4796-3352",
"affiliation": [
{
"name": "Faculty of Medicine, University of Melbourne , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "Department of Haematology, St Vincent's Hospital Melbourne , Fitzroy, Victoria ,",
"place": [
"Australia"
]
}
],
"authenticated-orcid": false,
"family": "Quach",
"given": "Hang",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-1605-7226",
"affiliation": [
{
"name": "Drug Delivery, Disposition and Dynamics, Monash Institute of Pharmaceutical Sciences, Monash University , Melbourne, Victoria ,",
"place": [
"Australia"
]
}
],
"authenticated-orcid": false,
"family": "McIntosh",
"given": "Michelle P",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Peter MacCallum Cancer Centre , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "Department of Infectious Diseases, Royal Melbourne Hospital , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "National Centre for Infections in Cancer, Peter MacCallum Cancer Centre , Melbourne, Victoria ,",
"place": [
"Australia"
]
}
],
"family": "Page",
"given": "Hayley",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Peter MacCallum Cancer Centre , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "Department of Infectious Diseases, Royal Melbourne Hospital , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "National Centre for Infections in Cancer, Peter MacCallum Cancer Centre , Melbourne, Victoria ,",
"place": [
"Australia"
]
}
],
"family": "Woolstencroft",
"given": "Rachel",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-8443-314X",
"affiliation": [
{
"name": "Department of Infectious Diseases, Peter MacCallum Cancer Centre , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "Sir Peter MacCallum Department of Oncology, The University of Melbourne , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "Department of Infectious Diseases, Royal Melbourne Hospital , Melbourne, Victoria ,",
"place": [
"Australia"
]
},
{
"name": "National Centre for Infections in Cancer, Peter MacCallum Cancer Centre , Melbourne, Victoria ,",
"place": [
"Australia"
]
}
],
"authenticated-orcid": false,
"family": "Slavin",
"given": "Monica",
"sequence": "additional"
}
],
"container-title": "Clinical Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
8,
28
]
],
"date-time": "2025-08-28T13:01:44Z",
"timestamp": 1756386104000
},
"deposited": {
"date-parts": [
[
2025,
8,
28
]
],
"date-time": "2025-08-28T13:01:48Z",
"timestamp": 1756386108000
},
"funder": [
{
"award": [
"2002213"
],
"name": "Australian Medical Research Future Fund Respiratory Medicine Clinical Trials Research"
},
{
"award": [
"1116876"
],
"name": "National Health Medical Research Council Centre of Research Excellence"
}
],
"indexed": {
"date-parts": [
[
2025,
8,
28
]
],
"date-time": "2025-08-28T13:40:01Z",
"timestamp": 1756388401366,
"version": "3.44.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
8,
28
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
8,
28
]
],
"date-time": "2025-08-28T00:00:00Z",
"timestamp": 1756339200000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciaf409/64135638/ciaf409.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciaf409/64135638/ciaf409.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2025,
8,
28
]
]
},
"published-online": {
"date-parts": [
[
2025,
8,
28
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"author": "World Health Organization (WHO)",
"key": "2025082809013909000_ciaf409-B1"
},
{
"DOI": "10.1093/cid/ciae308",
"article-title": "How immunocompromised hosts were left behind in the quest to control the COVID-19 pandemic",
"author": "Boeckh",
"doi-asserted-by": "crossref",
"first-page": "1018",
"journal-title": "Clin Infect Dis",
"key": "2025082809013909000_ciaf409-B2",
"volume": "79",
"year": "2024"
},
{
"article-title": "COVID-19 infection among patients with cancer in Australia from 2020 to 2022: a national multicentre cohort study",
"author": "Hall",
"first-page": "100824",
"journal-title": "Lancet Reg Health West Pac",
"key": "2025082809013909000_ciaf409-B3",
"volume": "38",
"year": "2023"
},
{
"DOI": "10.1016/j.lanepe.2023.100680",
"article-title": "Were cancer patients worse off than the general population during the COVID-19 pandemic? A population-based study from Norway, Denmark and Iceland during the pre-vaccination era",
"author": "Johansson",
"doi-asserted-by": "crossref",
"first-page": "100680",
"journal-title": "Lancet Reg Health Europe",
"key": "2025082809013909000_ciaf409-B4",
"volume": "31",
"year": "2023"
},
{
"DOI": "10.1186/s13045-021-01177-0",
"article-title": "COVID-19 infection in adult patients with hematological malignancies: a European Hematology Association Survey (EPICOVIDEHA)",
"author": "Pagano",
"doi-asserted-by": "crossref",
"first-page": "168",
"journal-title": "J Hematol Oncol",
"key": "2025082809013909000_ciaf409-B5",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1093/ofid/ofad550",
"article-title": "Evolution of humoral and cellular immunity post–breakthrough coronavirus disease 2019 in vaccinated patients with hematologic malignancy receiving tixagevimab-cilgavimab",
"author": "Hall",
"doi-asserted-by": "crossref",
"journal-title": "Open Forum Infect Dis",
"key": "2025082809013909000_ciaf409-B6",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1182/bloodadvances.2021006333",
"article-title": "Immunogenicity of COVID-19 vaccines in patients with hematologic malignancies: a systematic review and meta-analysis",
"author": "Teh",
"doi-asserted-by": "crossref",
"first-page": "2014",
"journal-title": "Blood Adv",
"key": "2025082809013909000_ciaf409-B7",
"volume": "6",
"year": "2022"
},
{
"DOI": "10.1080/10428194.2023.2224472",
"article-title": "Breakthrough COVID-19 is mild in vaccinated patients with hematological malignancy receiving tixagevimab-cilgavimab as pre-exposure prophylaxis",
"author": "Hall",
"doi-asserted-by": "crossref",
"first-page": "1600",
"journal-title": "Leuk Lymphoma",
"key": "2025082809013909000_ciaf409-B8",
"volume": "64",
"year": "2023"
},
{
"DOI": "10.1056/NEJMc2406750",
"article-title": "Influenza vaccination strategies in patients with hematologic cancer",
"author": "Hall",
"doi-asserted-by": "crossref",
"first-page": "306",
"journal-title": "N Engl J Med",
"key": "2025082809013909000_ciaf409-B9",
"volume": "392",
"year": "2025"
},
{
"DOI": "10.1126/science.abd4570",
"article-title": "Inborn errors of type I IFN immunity in patients with life-threatening COVID-19",
"author": "Zhang",
"doi-asserted-by": "crossref",
"journal-title": "Science",
"key": "2025082809013909000_ciaf409-B10",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1016/S2665-9913(21)00034-5",
"article-title": "Type I interferon, anti-interferon antibodies, and COVID-19",
"author": "Calabrese",
"doi-asserted-by": "crossref",
"first-page": "e246",
"journal-title": "Lancet Rheumatol",
"key": "2025082809013909000_ciaf409-B11",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.1016/j.antiviral.2020.104791",
"article-title": "Type 1 interferons as a potential treatment against COVID-19",
"author": "Sallard",
"doi-asserted-by": "crossref",
"first-page": "104791",
"journal-title": "Antiviral Res",
"key": "2025082809013909000_ciaf409-B12",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1056/NEJM198601093140202",
"article-title": "Prevention of natural colds by contact prophylaxis with intranasal alpha 2-interferon",
"author": "Hayden",
"doi-asserted-by": "crossref",
"first-page": "71",
"journal-title": "N Engl J Med",
"key": "2025082809013909000_ciaf409-B13",
"volume": "314",
"year": "1986"
},
{
"DOI": "10.1056/NEJM198601093140201",
"article-title": "Prophylactic efficacy of intranasal alpha 2-interferon against rhinovirus infections in the family setting",
"author": "Douglas",
"doi-asserted-by": "crossref",
"first-page": "65",
"journal-title": "N Engl J Med",
"key": "2025082809013909000_ciaf409-B14",
"volume": "314",
"year": "1986"
},
{
"DOI": "10.1089/jir.1980.1.169",
"article-title": "The preventive effect of human interferon-alpha preparation on upper respiratory disease",
"author": "Imanishi",
"doi-asserted-by": "crossref",
"first-page": "169",
"journal-title": "J Interferon Res",
"key": "2025082809013909000_ciaf409-B15",
"volume": "1",
"year": "1980"
},
{
"DOI": "10.1007/s10875-023-01452-4",
"article-title": "Interferon-α-2b nasal spray for treating SARS-CoV-2 omicron variant-infected children",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "862",
"journal-title": "J Clin Immunol",
"key": "2025082809013909000_ciaf409-B16",
"volume": "43",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2209760",
"article-title": "Early treatment with pegylated interferon lambda for Covid-19",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "518",
"journal-title": "N Engl J Med",
"key": "2025082809013909000_ciaf409-B17",
"volume": "388",
"year": "2023"
},
{
"DOI": "10.1093/jnci/djaa159",
"article-title": "Prevalence and outcome of COVID-19 infection in cancer patients: a national Veterans Affairs study",
"author": "Fillmore",
"doi-asserted-by": "crossref",
"first-page": "691",
"journal-title": "J Natl Cancer Inst",
"key": "2025082809013909000_ciaf409-B18",
"volume": "113",
"year": "2021"
},
{
"DOI": "10.1128/AAC.26.1.31",
"article-title": "Intranasal interferon-alpha 2 for prevention of natural rhinovirus colds",
"author": "Farr",
"doi-asserted-by": "crossref",
"first-page": "31",
"journal-title": "Antimicrob Agents Chemother",
"key": "2025082809013909000_ciaf409-B19",
"volume": "26",
"year": "1984"
},
{
"author": "Australian Bureau of Statistics",
"key": "2025082809013909000_ciaf409-B20",
"volume-title": "COVID-19 mortality by wave",
"year": "2022"
},
{
"DOI": "10.1016/j.vaccine.2010.03.062",
"article-title": "A randomized controlled trial of low-dose recombinant human interferons alpha-2b nasal spray to prevent acute viral respiratory infections in military recruits",
"author": "Gao",
"doi-asserted-by": "crossref",
"first-page": "4445",
"journal-title": "Vaccine",
"key": "2025082809013909000_ciaf409-B21",
"volume": "28",
"year": "2010"
},
{
"DOI": "10.2174/1568026621666210429083050",
"article-title": "The effect of recombinant human interferon alpha nasal drops to prevent COVID-19 pneumonia for medical staff in an epidemic area",
"author": "Meng",
"doi-asserted-by": "crossref",
"first-page": "920",
"journal-title": "Curr Top Med Chem",
"key": "2025082809013909000_ciaf409-B22",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1016/S2468-2667(22)00090-1",
"article-title": "Cancer incidence and mortality in Australia from 2020 to 2044 and an exploratory analysis of the potential effect of treatment delays during the COVID-19 pandemic: a statistical modelling study",
"author": "Luo",
"doi-asserted-by": "crossref",
"first-page": "e537",
"journal-title": "Lancet Public Health",
"key": "2025082809013909000_ciaf409-B23",
"volume": "7",
"year": "2022"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaf409/8241089"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Interferon-α Nasal Spray Prophylaxis Reduces COVID-19 in Cancer Patients: A Randomized, Double-Blinded, Placebo-Controlled Trial",
"type": "journal-article"
}