In Vitro Evaluation and Mitigation of Niclosamide’s Liabilities as a COVID-19 Treatment
et al., Vaccines, doi:10.3390/vaccines10081284, Aug 2022
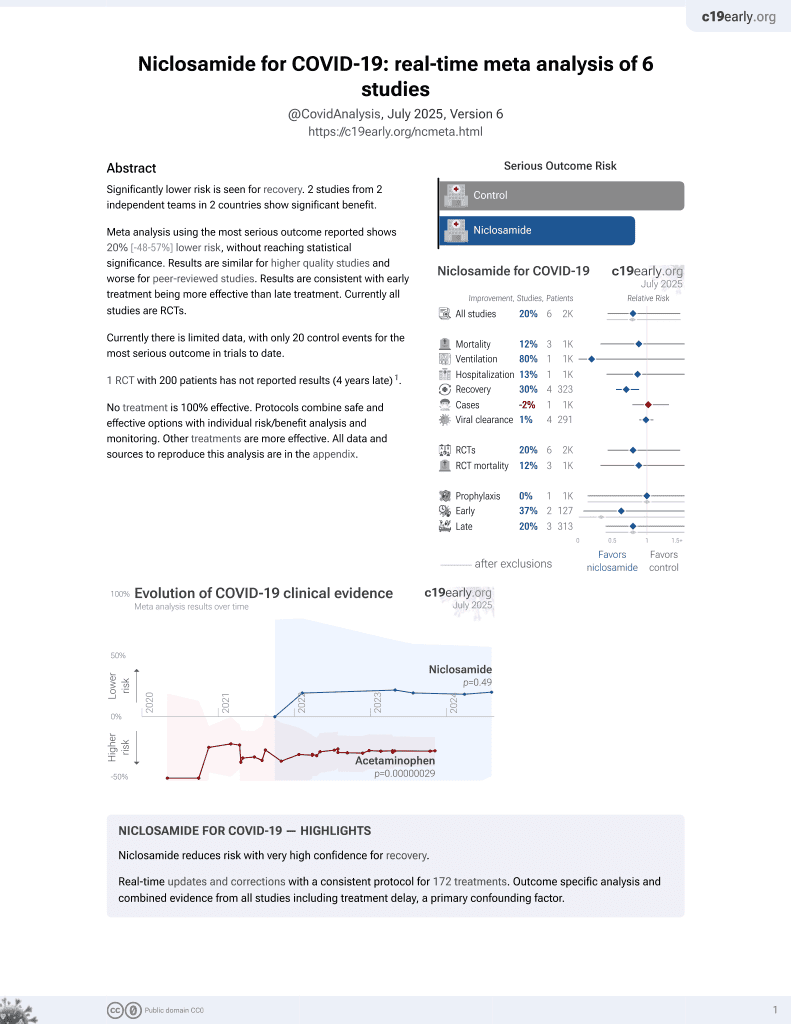
56th treatment shown to reduce risk in
August 2025, now with p = 0.0069 from 7 studies.
Lower risk for recovery.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
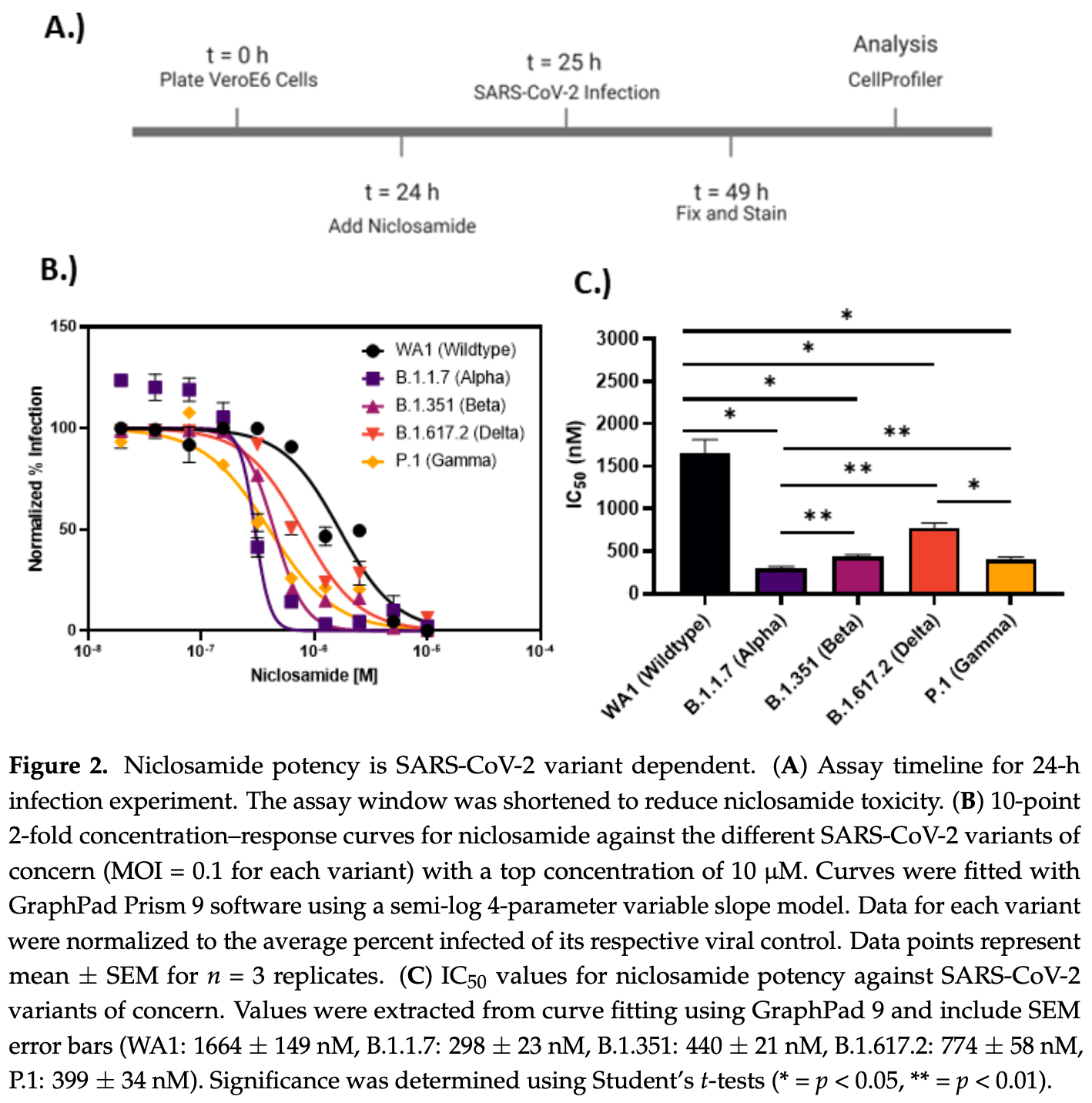
In vitro study suggesting that niclosamide has variable potency against SARS-CoV-2 variants and may have cytotoxicity concerns and significant polypharmacology. Authors found that niclosamide inhibited SARS-CoV-2 infection in VeroE6 and H1437 cells with IC50 values of 564 nM and 261 nM respectively, but had a poor selectivity index of 2. Imaging analysis suggested niclosamide inhibits viral entry and cell-to-cell spread via syncytia. Testing of 33 niclosamide analogs identified several with reduced cytotoxicity and improved potency.
9 preclinical studies support the efficacy of niclosamide for COVID-19:
In silico studies predict inhibition of SARS-CoV-2 with niclosamide or metabolites via binding to the spikeA,1, MproB,1, RNA-dependent RNA polymeraseC,1, PLproD,1, nucleocapsidE,1, and helicaseF,1 proteins.
Niclosamide inhibits endolysosomal acidification and suppresses
TLR3-mediated pro-inflammatory signaling in human small airway
epithelial cells stimulated with TLR3 agonists mimicking viral RNA2, modulates host lipid metabolism and reduces
infectious SARS-CoV-2 virion production in Vero E6 cells4, reduces CD147 protein levels and inhibits
SARS-CoV-2-induced upregulation of CD147 in A549-ACE2 cells, including
the highly glycosylated form of CD147 which has been implicated in
COVID-19 disease progression and post-COVID-19 cardiac complications5, blocked the formation of syncytia mediated by
SARS-CoV-2 spike protein pseudovirus-producing cells6, may reduce inflammation, NLRP3 formation, and
caspase-1 activity9, may inhibit viral uncoating, replication, and
assembly via disruption of pH gradients and reduced ATP production in
host cells8, may counter immune evasion by reversing E-, ORF7a-, and ORF8-mediated down-regulation of MHC-I, preserving CD8⁺ T-cell recognition10, and shows strong synergy when combined with
ivermectin7.
1.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
2.
Pejler et al., Blockade of endolysosomal acidification suppresses TLR3-mediated pro-inflammatory signaling in airway epithelial cells, Journal of Allergy and Clinical Immunology, doi:10.1016/j.jaci.2024.05.031.
3.
Walia et al., SARS-CoV-2 virulence factor ORF3a blocks lysosome function by modulating TBC1D5-dependent Rab7 GTPase cycle, Nature Communications, doi:10.1038/s41467-024-46417-2.
4.
Garrett et al., Niclosamide as a chemical probe for analyzing SARS-CoV-2 modulation of host cell lipid metabolism, Frontiers in Microbiology, doi:10.3389/fmicb.2023.1251065.
5.
Yang et al., Repurposing Niclosamide as a Novel Anti-SARS-CoV-2 Drug by Restricting Entry Protein CD147, Biomedicines, doi:10.3390/biomedicines11072019.
6.
Sheng et al., A pseudovirus-based method to dynamically mimic SARS-CoV-2-associated cell-to-cell fusion and transmission, Acta Biochimica et Biophysica Sinica, doi:10.3724/abbs.2023129.
7.
Jitobaom et al., Synergistic anti-SARS-CoV-2 activity of repurposed anti-parasitic drug combinations, BMC Pharmacology and Toxicology, doi:10.1186/s40360-022-00580-8.
8.
Needham, D., The pH Dependence of Niclosamide Solubility, Dissolution, and Morphology: Motivation for Potentially Universal Mucin-Penetrating Nasal and Throat Sprays for COVID19, its Variants and other Viral Infections, Pharmaceutical Research, doi:10.1007/s11095-021-03112-x.
a.
The trimeric spike (S) protein is a glycoprotein that mediates viral entry by binding to the host ACE2 receptor, is critical for SARS-CoV-2's ability to infect host cells, and is a target of neutralizing antibodies. Inhibition of the spike protein prevents viral attachment, halting infection at the earliest stage.
b.
The main protease or Mpro, also known as 3CLpro or nsp5, is a cysteine protease that cleaves viral polyproteins into functional units needed for replication. Inhibiting Mpro disrupts the SARS-CoV-2 lifecycle within the host cell, preventing the creation of new copies.
c.
RNA-dependent RNA polymerase (RdRp), also called nsp12, is the core enzyme of the viral replicase-transcriptase complex that copies the positive-sense viral RNA genome into negative-sense templates for progeny RNA synthesis. Inhibiting RdRp blocks viral genome replication and transcription.
d.
The papain-like protease (PLpro) has multiple functions including cleaving viral polyproteins and suppressing the host immune response by deubiquitination and deISGylation of host proteins. Inhibiting PLpro may block viral replication and help restore normal immune responses.
e.
The nucleocapsid (N) protein binds and encapsulates the viral genome by coating the viral RNA. N enables formation and release of infectious virions and plays additional roles in viral replication and pathogenesis. N is also an immunodominant antigen used in diagnostic assays.
f.
The helicase, or nsp13, protein unwinds the double-stranded viral RNA, a crucial step in replication and transcription. Inhibition may prevent viral genome replication and the creation of new virus components.
Wotring et al., 9 Aug 2022, peer-reviewed, 12 authors.
Contact: jzsexton@med.umich.edu (corresponding author).
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
In Vitro Evaluation and Mitigation of Niclosamide’s Liabilities as a COVID-19 Treatment
Vaccines, doi:10.3390/vaccines10081284
Niclosamide, an FDA-approved oral anthelmintic drug, has broad biological activity including anticancer, antibacterial, and antiviral properties. Niclosamide has also been identified as a potent inhibitor of SARS-CoV-2 infection in vitro, generating interest in its use for the treatment or prevention of COVID-19. Unfortunately, there are several potential issues with using niclosamide for COVID-19, including low bioavailability, significant polypharmacology, high cellular toxicity, and unknown efficacy against emerging SARS-CoV-2 variants of concern. In this study, we used high-content imaging-based immunofluorescence assays in two different cell models to assess these limitations and evaluate the potential for using niclosamide as a COVID-19 antiviral. We show that despite promising preliminary reports, the antiviral efficacy of niclosamide overlaps with its cytotoxicity giving it a poor in vitro selectivity index for anti-SARS-CoV-2 inhibition. We also show that niclosamide has significantly variable potency against the different SARS-CoV-2 variants of concern and is most potent against variants with enhanced cell-to-cell spread including the B.1.1.7 (alpha) variant. Finally, we report the activity of 33 niclosamide analogs, several of which have reduced cytotoxicity and increased potency relative to niclosamide. A preliminary structure-activity relationship analysis reveals dependence on a protonophore for antiviral efficacy, which implicates nonspecific endolysosomal neutralization as a dominant mechanism of action. Further single-cell morphological profiling suggests niclosamide also inhibits viral entry and cell-to-cell spread by syncytia. Altogether, our results suggest that niclosamide is not an ideal candidate for the treatment of COVID-19, but that there is potential for developing improved analogs with higher clinical translational potential in the future.
Supplementary Materials: The following supporting information can be downloaded at: https: //www.mdpi.com/article/10.3390/vaccines10081284/s1 , Supplementary Figure S1 : Control data for VeroE6 and H1437 SARS-CoV-2 long-term exposure bioassays; Supplementary Figure S2: Niclosamide analog structures; Supplementary Figure S3 : Some niclosamide analogs cause dose-dependent exacerbation of infection in H1437. Author Contributions: Conceptualization, J.W.W., S.M.M., J.Z.S. and M.J.O.; methodology, J.W.W., S.M.M. and C.M.; formal analysis, J.W.W., S.M.M., M.C.C. and J.Z.S., writing-original draft preparation, J.W.W., S.M.M., K.S. and J.Z.S.; writing-review and editing, J.W.W., S.M.M., K.S., C.J.Z., T.N., S.R.M., R.F., C.M., M.C.C., C.E.W., M.J.O. and J.Z.S.; visualization, J.W.W., S.M.M. and J.Z.S.; supervision, J.Z.S., M.C.C., C.E.W. and M.J.O.; project administration, J.Z.S.; funding acquisition, J.Z.S. All authors have read and agreed to the published version of the manuscript. Funding: This research was funded by the National Center for Advancing Translational Science grant number UL1TR002240. The APC was funded by the same grant. Institutional Review Board Statement: This work was performed under the approval of the University of Michigan Institutional Biosafety Committee under protocol IBCA00001528.
Informed Consent Statement: Not applicable. Data Availability Statement: All relevant data are within the paper and its Supplementary Materials files.
Conflicts of..
References
Aslan, Aslan, Zolbanin, Jafari, Acute respiratory distress syndrome in COVID-19: Possible mechanisms and therapeutic management, Pneumonia
Backer, Sjöbring, Sonne, Weiss, Hostrup et al., A randomized, double-blind, placebo-controlled phase 1 trial of inhaled and intranasal niclosamide: A broad spectrum antiviral candidate for treatment of COVID-19, Lancet Reg. Health-Eur, doi:10.1016/j.lanepe.2021.100084
Berthold, Cebron, Dill, Gabriel, Kötter et al., KNIME: The Konstanz information miner, ACM SIGKDD Explor. Newsl, doi:10.1145/1656274.1656280
Bhagat, Compton, Musso, Laudeman, Jackson et al., N-substituted phenylbenzamides of the niclosamide chemotype attenuate obesity related changes in high fat diet fed mice, PLoS ONE, doi:10.1371/journal.pone.0204605
Blaikie, Brown, Samuelsson, Brand, Smith et al., Targeting dinitrophenol to mitochondria: Limitations to the development of a self-limiting mitochondrial protonophore, Biosci Rep, doi:10.1007/s10540-006-9018-8
Blake, Shaabani, Eubanks, Maruyama, Manning et al., Salicylanilides Reduce SARS-CoV-2 Replication and Suppress Induction of Inflammatory Cytokines in a Rodent Model, ACS Infect. Dis, doi:10.1021/acsinfecdis.1c00253
Braga, Ali, Secco, Chiavacci, Neves et al., Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia, Nature, doi:10.1038/s41586-021-03491-6
Bussani, Schneider, Zentilin, Collesi, Ali et al., Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology, EBioMedicine, doi:10.1016/j.ebiom.2020.103104
Cairns, Dulko, Griffiths, Golan, Cohen et al., Efficacy of Niclosamide vs Placebo in SARS-CoV-2 Respiratory Viral Clearance, Viral Shedding, and Duration of Symptoms Among Patients with Mild to Moderate COVID-19: A Phase 2 Randomized Clinical Trial, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2021.44942
Chang, Yeh, Lin, Chen, Yao et al., Pharmacokinetics of anti-SARS-CoV agent niclosamide and its analogs in rats, J. Food Drug Anal, doi:10.38212/2224-6614.2464
Chen, Mook, Premont, Wang, Niclosamide: Beyond an antihelminthic drug, Cell. Signal, doi:10.1016/j.cellsig.2017.04.001
Chen, Zhang, Case, Winkler, Liu et al., Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies, Nat. Med, doi:10.1038/s41591-021-01294-w
Cully, A tale of two antiviral targets-and the COVID-19 drugs that bind them, Nat. Rev. Drug Discov, doi:10.1038/d41573-021-00202-8
Dong, Du, Gardner, An interactive web-based dashboard to track COVID-19 in real time, Lancet Infect. Dis, doi:10.1016/S1473-3099(20)30120-1
Drayman, Demarco, Jones, Azizi, Froggatt et al., Masitinib is a broad coronavirus 3CL inhibitor that blocks replication of SARS-CoV-2, Science, doi:10.1126/science.abg5827
Fan, Li, Ding, Zhang, Contributions of Hepatic and Intestinal Metabolism to the Disposition of Niclosamide, a Repurposed Drug with Poor Bioavailability, Drug Metab. Dispos, doi:10.1124/dmd.119.086678
Feinmann, COVID-19: Global vaccine production is a mess and shortages are down to more than just hoarding, BMJ, doi:10.1136/bmj.n2375
Fonseca, Diering, Bidinosti, Dalal, Alain et al., Structure-activity analysis of niclosamide reveals potential role for cytoplasmic pH in control of mammalian target of rapamycin complex 1 (mTORC1) signaling, J. Biol. Chem, doi:10.1074/jbc.M112.359638
Gassen, Niemeyer, Muth, Corman, Martinelli et al., SARS-CoV-2-mediated dysregulation of metabolism and autophagy uncovers host-targeting antivirals, Nat. Commun, doi:10.1038/s41467-021-24007-w
Gottlieb, Vaca, Paredes, Mera, Webb et al., Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients, N. Engl. J. Med, doi:10.1056/NEJMoa2116846
Hamdoun, Jung, Efferth, Drug repurposing of the anthelmintic niclosamide to treat multidrug-resistant leukemia, Front. Pharmacol, doi:10.3389/fphar.2017.00110
Hammond, Leister-Tebbe, Gardner, Abreu, Bao et al., Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19, N. Engl. J. Med, doi:10.1056/NEJMoa2118542
Harvey, Carabelli, Jackson, Gupta, Thomson et al., SARS-CoV-2 variants, spike mutations and immune escape, Nat. Rev. Microbiol, doi:10.1038/s41579-021-00573-0
Hu, Guo, Zhou, Shi, Characteristics of SARS-CoV-2 and COVID-19, Nat. Rev. Microbiol, doi:10.1038/s41579-020-00459-7
Jeon, Ko, Lee, Choi, Byun et al., Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs, Antimicrob. Agents Chemother, doi:10.1128/AAC.00819-20
Jurgeit, Mcdowell, Moese, Meldrum, Schwendener et al., Niclosamide Is a Proton Carrier and Targets Acidic Endosomes with Broad Antiviral Effects, PLoS Pathog, doi:10.1371/journal.ppat.1002976
Kotova, Antonenko, Fifty Years of Research on Protonophores: Mitochondrial Uncoupling as a Basis for Therapeutic Action, Acta Nat, doi:10.32607/actanaturae.11610
Kumar, Coronel, Somalanka, Raju, Aning et al., Mitochondrial uncoupling reveals a novel therapeutic opportunity for p53-defective cancers, Nat. Commun, doi:10.1038/s41467-018-05805-1
Kyriakidis, López-Cortés, González, Grimaldos, Prado, SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates, Nat. Rev. Microbiol, doi:10.1038/s41541-021-00292-w
Lin, Bai, Hu, Wang, Chao et al., Preclinical evaluation of a nanoformulated antihelminthic, niclosamide, in ovarian cancer, Oncotarget, doi:10.18632/oncotarget.7113
Lin, Li, Wang, Shi, Syncytia formation during SARS-CoV-2 lung infection: A disastrous unity to eliminate lymphocytes, Cell Death Differ, doi:10.1038/s41418-021-00795-y
Liu, Wei, Kappler, Marrack, Zhang, SARS-CoV-2 Variants of Concern and Variants of Interest Receptor Binding Domain Mutations and Virus Infectivity, Front. Immunol, doi:10.3389/fimmu.2022.825256
Mcquin, Goodman, Chernyshev, Kamentsky, Cimini et al., CellProfiler 3.0: Next-generation image processing for biology, PLoS Biol, doi:10.1371/journal.pbio.2005970
Mirabelli, Wotring, Zhang, Mccarty, Fursmidt et al., Morphological cell profiling of SARS-CoV-2 infection identifies drug repurposing candidates for COVID-19, Proc. Natl. Acad. Sci, doi:10.1073/pnas.2105815118
Mizrahi, Shilo, Rossman, Kalkstein, Marcus et al., Longitudinal symptom dynamics of COVID-19 infection, Nat. Commun, doi:10.1038/s41467-020-20053-y
Parikh, Liu, Wu, Evans, Dall'era et al., Phase Ib trial of reformulated niclosamide with abiraterone/prednisone in men with castration-resistant prostate cancer, Sci. Rep, doi:10.1038/s41598-021-85969-x
Park, Jo, Pak, Bae, Rhim et al., FCCP depolarizes plasma membrane potential by activating proton and Na+ currents in bovine aortic endothelial cells, Pflug. Arch, doi:10.1007/s004240100703
Prabhakara, Godbole, Sil, Jahnavi, Gulzar et al., Strategies to target SARS-CoV-2 entry and infection using dual mechanisms of inhibition by acidification inhibitors, PLoS Pathog, doi:10.1371/journal.ppat.1009706
Pushpakom, Iorio, Eyers, Escott, Hopper et al., Drug repurposing: Progress, challenges and recommendations, Nat. Rev. Drug Discov, doi:10.1038/nrd.2018.168
Qomara, Primanissa, Amalia, Purwadi, Zakiyah, Effectiveness of Remdesivir, Lopinavir/Ritonavir, and Favipiravir for COVID-19 Treatment: A Systematic Review, Int. J. Gen. Med, doi:10.2147/IJGM.S332458
Rajah, Bernier, Buchrieser, Schwartz, The Mechanism and Consequences of SARS-CoV-2 Spike-Mediated Fusion and Syncytia Formation, J. Mol. Biol, doi:10.1016/j.jmb.2021.167280
Rajah, Hubert, Bishop, Saunders, Robinot et al., SARS-CoV-2 Alpha, Beta, and Delta variants display enhanced Spike-mediated syncytia formation, EMBO J, doi:10.15252/embj.2021108944
Reddy, Zhang, Polypharmacology: Drug discovery for the future, Expert Rev. Clin. Pharmacol, doi:10.1586/ecp.12.74
Reed, Muench, A simple method of estimating fifty per cent endpoints, Am. J. Epidemiol, doi:10.1093/oxfordjournals.aje.a118408
Saito, Irie, Suzuki, Maemura, Nasser et al., Enhanced fusogenicity and pathogenicity of SARS-CoV-2 Delta P681R mutation, Nature, doi:10.1038/s41586-021-04266-9
Shrestha, Johnson, Byrne, Exploring the therapeutic potential of mitochondrial uncouplers in cancer, Mol. Metab, doi:10.1016/j.molmet.2021.101222
Takashita, Kinoshita, Yamayoshi, Sakai-Tagawa, Fujisaki et al., Efficacy of Antiviral Agents against the SARS-CoV-2 Omicron Subvariant BA.2, N. Engl. J. Med, doi:10.1056/NEJMc2201933
Touret, Gilles, Barral, Nougairède, Van Helden et al., In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication, Sci. Rep, doi:10.1038/s41598-020-70143-6
Weiss, Touret, Baronti, Gilles, Hoen et al., Niclosamide shows strong antiviral activity in a human airway model of SARS-CoV-2 infection and a conserved potency against the Alpha (B.1.1.7), Beta (B.1.351) and Delta variant (B.1.617, PLoS ONE, doi:10.1371/journal.pone.0260958
Whitley, Molnupiravir-A Step toward Orally Bioavailable Therapies for COVID-19, N. Engl. J. Med, doi:10.1056/NEJMe2117814
Wieland, Trageser, Gogolok, Reinartz, Höfer et al., Anticancer effects of niclosamide in human glioblastoma, Clin. Cancer Res, doi:10.1158/1078-0432.CCR-12-2895
Wotring, Fursmidt, Ward, Sexton, Evaluating the in vitro efficacy of bovine lactoferrin products against SARS-CoV-2 variants of concern, J. Dairy Sci, doi:10.3168/jds.2021-21247
Wu, Jan, Chen, Hsieh, Hwang et al., Inhibition of Severe Acute Respiratory Syndrome Coronavirus Replication by Niclosamide, Antimicrob. Agents Chemother, doi:10.1128/AAC.48.7.2693-2696.2004
Xiao, Wang, Chang, Wang, Dong et al., Identification of Potent and Safe Antiviral Therapeutic Candidates Against SARS-CoV-2, Front. Immunol, doi:10.3389/fimmu.2020.586572
Zhang, Zhang, Fang, Liu, Ye et al., SARS-CoV-2 spike L452R mutation increases Omicron variant fusogenicity and infectivity as well as host glycolysis, Signal Transduct. Target. Ther, doi:10.1038/s41392-022-00941-z
DOI record:
{
"DOI": "10.3390/vaccines10081284",
"ISSN": [
"2076-393X"
],
"URL": "http://dx.doi.org/10.3390/vaccines10081284",
"abstract": "<jats:p>Niclosamide, an FDA-approved oral anthelmintic drug, has broad biological activity including anticancer, antibacterial, and antiviral properties. Niclosamide has also been identified as a potent inhibitor of SARS-CoV-2 infection in vitro, generating interest in its use for the treatment or prevention of COVID-19. Unfortunately, there are several potential issues with using niclosamide for COVID-19, including low bioavailability, significant polypharmacology, high cellular toxicity, and unknown efficacy against emerging SARS-CoV-2 variants of concern. In this study, we used high-content imaging-based immunofluorescence assays in two different cell models to assess these limitations and evaluate the potential for using niclosamide as a COVID-19 antiviral. We show that despite promising preliminary reports, the antiviral efficacy of niclosamide overlaps with its cytotoxicity giving it a poor in vitro selectivity index for anti-SARS-CoV-2 inhibition. We also show that niclosamide has significantly variable potency against the different SARS-CoV-2 variants of concern and is most potent against variants with enhanced cell-to-cell spread including the B.1.1.7 (alpha) variant. Finally, we report the activity of 33 niclosamide analogs, several of which have reduced cytotoxicity and increased potency relative to niclosamide. A preliminary structure–activity relationship analysis reveals dependence on a protonophore for antiviral efficacy, which implicates nonspecific endolysosomal neutralization as a dominant mechanism of action. Further single-cell morphological profiling suggests niclosamide also inhibits viral entry and cell-to-cell spread by syncytia. Altogether, our results suggest that niclosamide is not an ideal candidate for the treatment of COVID-19, but that there is potential for developing improved analogs with higher clinical translational potential in the future.</jats:p>",
"alternative-id": [
"vaccines10081284"
],
"author": [
{
"affiliation": [
{
"name": "Department of Medicinal Chemistry, College of Pharmacy, University of Michigan, Ann Arbor, MI 48109, USA"
}
],
"family": "Wotring",
"given": "Jesse W.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Medicinal Chemistry, College of Pharmacy, University of Michigan, Ann Arbor, MI 48109, USA"
}
],
"family": "McCarty",
"given": "Sean M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicinal Chemistry, College of Pharmacy, University of Michigan, Ann Arbor, MI 48109, USA"
}
],
"family": "Shafiq",
"given": "Khadija",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0300-849X",
"affiliation": [
{
"name": "Department of Medicinal Chemistry, College of Pharmacy, University of Michigan, Ann Arbor, MI 48109, USA"
}
],
"authenticated-orcid": false,
"family": "Zhang",
"given": "Charles J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Gastroenterology and Hepatology, Michigan Medicine at the University of Michigan, Ann Arbor, MI 48109, USA"
}
],
"family": "Nguyen",
"given": "Theophilus",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicinal Chemistry, College of Pharmacy, University of Michigan, Ann Arbor, MI 48109, USA"
}
],
"family": "Meyer",
"given": "Sophia R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Gastroenterology and Hepatology, Michigan Medicine at the University of Michigan, Ann Arbor, MI 48109, USA"
}
],
"family": "Fursmidt",
"given": "Reid",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4785-5482",
"affiliation": [
{
"name": "Department of Microbiology and Immunology, University of Michigan, Ann Arbor, MI 48109, USA"
}
],
"authenticated-orcid": false,
"family": "Mirabelli",
"given": "Carmen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicinal Chemistry, College of Pharmacy, University of Michigan, Ann Arbor, MI 48109, USA"
}
],
"family": "Clasby",
"given": "Martin C.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5286-0924",
"affiliation": [
{
"name": "Department of Microbiology and Immunology, University of Michigan, Ann Arbor, MI 48109, USA"
}
],
"authenticated-orcid": false,
"family": "Wobus",
"given": "Christiane E.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3128-5331",
"affiliation": [
{
"name": "Department of Computational Medicine and Bioinformatics, University of Michigan, Ann Arbor, MI 48109, USA"
}
],
"authenticated-orcid": false,
"family": "O’Meara",
"given": "Matthew J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicinal Chemistry, College of Pharmacy, University of Michigan, Ann Arbor, MI 48109, USA"
},
{
"name": "Department of Internal Medicine, Gastroenterology and Hepatology, Michigan Medicine at the University of Michigan, Ann Arbor, MI 48109, USA"
},
{
"name": "U-M Center for Drug Repurposing, University of Michigan, Ann Arbor, MI 48109, USA"
}
],
"family": "Sexton",
"given": "Jonathan Z.",
"sequence": "additional"
}
],
"container-title": "Vaccines",
"container-title-short": "Vaccines",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
8,
10
]
],
"date-time": "2022-08-10T13:47:06Z",
"timestamp": 1660139226000
},
"deposited": {
"date-parts": [
[
2024,
8,
3
]
],
"date-time": "2024-08-03T17:13:13Z",
"timestamp": 1722705193000
},
"funder": [
{
"DOI": "10.13039/100006108",
"award": [
"UL1TR002240"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100006108",
"id-type": "DOI"
}
],
"name": "National Center for Advancing Translational Science"
}
],
"indexed": {
"date-parts": [
[
2024,
8,
4
]
],
"date-time": "2024-08-04T00:11:56Z",
"timestamp": 1722730316733
},
"is-referenced-by-count": 2,
"issue": "8",
"issued": {
"date-parts": [
[
2022,
8,
9
]
]
},
"journal-issue": {
"issue": "8",
"published-online": {
"date-parts": [
[
2022,
8
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
9
]
],
"date-time": "2022-08-09T00:00:00Z",
"timestamp": 1660003200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2076-393X/10/8/1284/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1284",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
8,
9
]
]
},
"published-online": {
"date-parts": [
[
2022,
8,
9
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1038/s41579-020-00459-7",
"article-title": "Characteristics of SARS-CoV-2 and COVID-19",
"author": "Hu",
"doi-asserted-by": "crossref",
"first-page": "141",
"journal-title": "Nat. Rev. Microbiol.",
"key": "ref_1",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1186/s41479-021-00092-9",
"article-title": "Acute respiratory distress syndrome in COVID-19: Possible mechanisms and therapeutic management",
"author": "Aslan",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Pneumonia",
"key": "ref_2",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1038/s41467-020-20053-y",
"article-title": "Longitudinal symptom dynamics of COVID-19 infection",
"author": "Mizrahi",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Nat. Commun.",
"key": "ref_3",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30120-1",
"article-title": "An interactive web-based dashboard to track COVID-19 in real time",
"author": "Dong",
"doi-asserted-by": "crossref",
"first-page": "533",
"journal-title": "Lancet Infect. Dis.",
"key": "ref_4",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1038/s41579-021-00573-0",
"article-title": "SARS-CoV-2 variants, spike mutations and immune escape",
"author": "Harvey",
"doi-asserted-by": "crossref",
"first-page": "409",
"journal-title": "Nat. Rev. Microbiol.",
"key": "ref_5",
"volume": "19",
"year": "2021"
},
{
"article-title": "SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates",
"author": "Kyriakidis",
"first-page": "1",
"journal-title": "Nat. Rev. Microbiol.",
"key": "ref_6",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1038/d41573-021-00202-8",
"article-title": "A tale of two antiviral targets-and the COVID-19 drugs that bind them",
"author": "Cully",
"doi-asserted-by": "crossref",
"first-page": "3",
"journal-title": "Nat. Rev. Drug Discov.",
"key": "ref_7",
"volume": "21",
"year": "2022"
},
{
"DOI": "10.1056/NEJMe2117814",
"article-title": "Molnupiravir—A Step toward Orally Bioavailable Therapies for COVID-19",
"author": "Whitley",
"doi-asserted-by": "crossref",
"first-page": "592",
"journal-title": "N. Engl. J. Med.",
"key": "ref_8",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N. Engl. J. Med.",
"key": "ref_9",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1136/bmj.n2375",
"article-title": "COVID-19: Global vaccine production is a mess and shortages are down to more than just hoarding",
"author": "Feinmann",
"doi-asserted-by": "crossref",
"first-page": "n2375",
"journal-title": "BMJ",
"key": "ref_10",
"volume": "375",
"year": "2021"
},
{
"DOI": "10.2147/IJGM.S332458",
"article-title": "Effectiveness of Remdesivir, Lopinavir/Ritonavir, and Favipiravir for COVID-19 Treatment: A Systematic Review",
"author": "Qomara",
"doi-asserted-by": "crossref",
"first-page": "8557",
"journal-title": "Int. J. Gen. Med.",
"key": "ref_11",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116846",
"article-title": "Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "305",
"journal-title": "N. Engl. J. Med.",
"key": "ref_12",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/s41591-021-01294-w",
"article-title": "Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "717",
"journal-title": "Nat. Med.",
"key": "ref_13",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1056/NEJMc2201933",
"article-title": "Efficacy of Antiviral Agents against the SARS-CoV-2 Omicron Subvariant BA.2",
"author": "Takashita",
"doi-asserted-by": "crossref",
"first-page": "1475",
"journal-title": "N. Engl. J. Med.",
"key": "ref_14",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/nrd.2018.168",
"article-title": "Drug repurposing: Progress, challenges and recommendations",
"author": "Pushpakom",
"doi-asserted-by": "crossref",
"first-page": "41",
"journal-title": "Nat. Rev. Drug Discov.",
"key": "ref_15",
"volume": "18",
"year": "2018"
},
{
"DOI": "10.1126/science.abg5827",
"article-title": "Masitinib is a broad coronavirus 3CL inhibitor that blocks replication of SARS-CoV-2",
"author": "Drayman",
"doi-asserted-by": "crossref",
"first-page": "931",
"journal-title": "Science",
"key": "ref_16",
"volume": "373",
"year": "2021"
},
{
"DOI": "10.1073/pnas.2105815118",
"article-title": "Morphological cell profiling of SARS-CoV-2 infection identifies drug repurposing candidates for COVID-19",
"author": "Mirabelli",
"doi-asserted-by": "crossref",
"first-page": "e2105815118",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_17",
"volume": "118",
"year": "2021"
},
{
"DOI": "10.1038/s41598-020-70143-6",
"article-title": "In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication",
"author": "Touret",
"doi-asserted-by": "crossref",
"first-page": "13093",
"journal-title": "Sci. Rep.",
"key": "ref_18",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2020.586572",
"article-title": "Identification of Potent and Safe Antiviral Therapeutic Candidates Against SARS-CoV-2",
"author": "Xiao",
"doi-asserted-by": "crossref",
"first-page": "586572",
"journal-title": "Front. Immunol.",
"key": "ref_19",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1128/AAC.00819-20",
"article-title": "Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs",
"author": "Jeon",
"doi-asserted-by": "crossref",
"first-page": "e00819-20",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_20",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1016/j.cellsig.2017.04.001",
"article-title": "Niclosamide: Beyond an antihelminthic drug",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "89",
"journal-title": "Cell. Signal.",
"key": "ref_21",
"volume": "41",
"year": "2018"
},
{
"DOI": "10.1074/jbc.M112.359638",
"article-title": "Structure-activity analysis of niclosamide reveals potential role for cytoplasmic pH in control of mammalian target of rapamycin complex 1 (mTORC1) signaling",
"author": "Fonseca",
"doi-asserted-by": "crossref",
"first-page": "17530",
"journal-title": "J. Biol. Chem.",
"key": "ref_22",
"volume": "287",
"year": "2012"
},
{
"DOI": "10.1038/s41467-018-05805-1",
"article-title": "Mitochondrial uncoupling reveals a novel therapeutic opportunity for p53-defective cancers",
"author": "Kumar",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Nat. Commun.",
"key": "ref_23",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1371/journal.ppat.1002976",
"doi-asserted-by": "crossref",
"key": "ref_24",
"unstructured": "Jurgeit, A., McDowell, R., Moese, S., Meldrum, E., Schwendener, R., and Greber, U.F. (2012). Niclosamide Is a Proton Carrier and Targets Acidic Endosomes with Broad Antiviral Effects. PLoS Pathog., 8."
},
{
"DOI": "10.1128/AAC.48.7.2693-2696.2004",
"article-title": "Inhibition of Severe Acute Respiratory Syndrome Coronavirus Replication by Niclosamide",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "2693",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_25",
"volume": "48",
"year": "2004"
},
{
"DOI": "10.1371/journal.ppat.1009706",
"doi-asserted-by": "crossref",
"key": "ref_26",
"unstructured": "Prabhakara, C., Godbole, R., Sil, P., Jahnavi, S., Gulzar, S.J., van Zanten, T.S., Sheth, D., Subhash, N., Chandra, A., and Shivaraj, A. (2021). Strategies to target SARS-CoV-2 entry and infection using dual mechanisms of inhibition by acidification inhibitors. PLoS Pathog., 17."
},
{
"DOI": "10.1038/s41467-021-24007-w",
"article-title": "SARS-CoV-2-mediated dysregulation of metabolism and autophagy uncovers host-targeting antivirals",
"author": "Gassen",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Nat. Commun.",
"key": "ref_27",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03491-6",
"article-title": "Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia",
"author": "Braga",
"doi-asserted-by": "crossref",
"first-page": "88",
"journal-title": "Nature",
"key": "ref_28",
"volume": "594",
"year": "2021"
},
{
"DOI": "10.18632/oncotarget.7113",
"article-title": "Preclinical evaluation of a nanoformulated antihelminthic, niclosamide, in ovarian cancer",
"author": "Lin",
"doi-asserted-by": "crossref",
"first-page": "8993",
"journal-title": "Oncotarget",
"key": "ref_29",
"volume": "7",
"year": "2016"
},
{
"DOI": "10.1124/dmd.119.086678",
"article-title": "Contributions of Hepatic and Intestinal Metabolism to the Disposition of Niclosamide, a Repurposed Drug with Poor Bioavailability",
"author": "Fan",
"doi-asserted-by": "crossref",
"first-page": "756",
"journal-title": "Drug Metab. Dispos.",
"key": "ref_30",
"volume": "47",
"year": "2019"
},
{
"article-title": "Pharmacokinetics of anti-SARS-CoV agent niclosamide and its analogs in rats",
"author": "Chang",
"first-page": "15",
"journal-title": "J. Food Drug Anal.",
"key": "ref_31",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1586/ecp.12.74",
"article-title": "Polypharmacology: Drug discovery for the future",
"author": "Reddy",
"doi-asserted-by": "crossref",
"first-page": "41",
"journal-title": "Expert Rev. Clin. Pharmacol.",
"key": "ref_32",
"volume": "6",
"year": "2013"
},
{
"DOI": "10.1158/1078-0432.CCR-12-2895",
"article-title": "Anticancer effects of niclosamide in human glioblastoma",
"author": "Wieland",
"doi-asserted-by": "crossref",
"first-page": "4124",
"journal-title": "Clin. Cancer Res.",
"key": "ref_33",
"volume": "19",
"year": "2013"
},
{
"DOI": "10.3389/fphar.2017.00110",
"article-title": "Drug repurposing of the anthelmintic niclosamide to treat multidrug-resistant leukemia",
"author": "Hamdoun",
"doi-asserted-by": "crossref",
"first-page": "110",
"journal-title": "Front. Pharmacol.",
"key": "ref_34",
"volume": "8",
"year": "2017"
},
{
"DOI": "10.15252/embj.2021108944",
"article-title": "SARS-CoV-2 Alpha, Beta, and Delta variants display enhanced Spike-mediated syncytia formation",
"author": "Rajah",
"doi-asserted-by": "crossref",
"first-page": "e108944",
"journal-title": "EMBO J.",
"key": "ref_35",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-04266-9",
"article-title": "Enhanced fusogenicity and pathogenicity of SARS-CoV-2 Delta P681R mutation",
"author": "Saito",
"doi-asserted-by": "crossref",
"first-page": "300",
"journal-title": "Nature",
"key": "ref_36",
"volume": "602",
"year": "2022"
},
{
"article-title": "SARS-CoV-2 spike L452R mutation increases Omicron variant fusogenicity and infectivity as well as host glycolysis",
"author": "Zhang",
"first-page": "1",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "ref_37",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0204605",
"doi-asserted-by": "crossref",
"key": "ref_38",
"unstructured": "Bhagat, H.A., Compton, S.A., Musso, D.L., Laudeman, C.P., Jackson, K.M., Yi, N.Y., Nierobisz, L.S., Forsberg, L., Brenman, J.E., and Sexton, J.Z. (2018). N-substituted phenylbenzamides of the niclosamide chemotype attenuate obesity related changes in high fat diet fed mice. PLoS ONE, 13."
},
{
"DOI": "10.1007/s004240100703",
"article-title": "FCCP depolarizes plasma membrane potential by activating proton and Na+ currents in bovine aortic endothelial cells",
"author": "Park",
"doi-asserted-by": "crossref",
"first-page": "344",
"journal-title": "Pflug. Arch.",
"key": "ref_39",
"volume": "443",
"year": "2002"
},
{
"DOI": "10.1007/s10540-006-9018-8",
"article-title": "Targeting dinitrophenol to mitochondria: Limitations to the development of a self-limiting mitochondrial protonophore",
"author": "Blaikie",
"doi-asserted-by": "crossref",
"first-page": "231",
"journal-title": "Biosci Rep.",
"key": "ref_40",
"volume": "26",
"year": "2006"
},
{
"DOI": "10.1016/j.molmet.2021.101222",
"article-title": "Exploring the therapeutic potential of mitochondrial uncouplers in cancer",
"author": "Shrestha",
"doi-asserted-by": "crossref",
"first-page": "101222",
"journal-title": "Mol. Metab.",
"key": "ref_41",
"volume": "51",
"year": "2021"
},
{
"DOI": "10.32607/actanaturae.11610",
"article-title": "Fifty Years of Research on Protonophores: Mitochondrial Uncoupling as a Basis for Therapeutic Action",
"author": "Kotova",
"doi-asserted-by": "crossref",
"first-page": "4",
"journal-title": "Acta Nat.",
"key": "ref_42",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1021/acsinfecdis.1c00253",
"article-title": "Salicylanilides Reduce SARS-CoV-2 Replication and Suppress Induction of Inflammatory Cytokines in a Rodent Model",
"author": "Blake",
"doi-asserted-by": "crossref",
"first-page": "2229",
"journal-title": "ACS Infect. Dis.",
"key": "ref_43",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2021.44942",
"article-title": "Efficacy of Niclosamide vs Placebo in SARS-CoV-2 Respiratory Viral Clearance, Viral Shedding, and Duration of Symptoms Among Patients with Mild to Moderate COVID-19: A Phase 2 Randomized Clinical Trial",
"author": "Cairns",
"doi-asserted-by": "crossref",
"first-page": "e2144942",
"journal-title": "JAMA Netw. Open",
"key": "ref_44",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1038/s41598-021-85969-x",
"article-title": "Phase Ib trial of reformulated niclosamide with abiraterone/prednisone in men with castration-resistant prostate cancer",
"author": "Parikh",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Sci. Rep.",
"key": "ref_45",
"volume": "11",
"year": "2021"
},
{
"article-title": "A randomized, double-blind, placebo-controlled phase 1 trial of inhaled and intranasal niclosamide: A broad spectrum antiviral candidate for treatment of COVID-19",
"author": "Backer",
"first-page": "100084",
"journal-title": "Lancet Reg. Health-Eur.",
"key": "ref_46",
"volume": "4",
"year": "2021"
},
{
"article-title": "SARS-CoV-2 Variants of Concern and Variants of Interest Receptor Binding Domain Mutations and Virus Infectivity",
"author": "Liu",
"first-page": "50",
"journal-title": "Front. Immunol.",
"key": "ref_47",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0260958",
"doi-asserted-by": "crossref",
"key": "ref_48",
"unstructured": "Weiss, A., Touret, F., Baronti, C., Gilles, M., Hoen, B., Nougairède, A., de Lamballerie, X., and Sommer, M.O. (2021). Niclosamide shows strong antiviral activity in a human airway model of SARS-CoV-2 infection and a conserved potency against the Alpha (B.1.1.7), Beta (B.1.351) and Delta variant (B.1.617.2). PLoS ONE, 16."
},
{
"DOI": "10.1016/j.ebiom.2020.103104",
"article-title": "Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology",
"author": "Bussani",
"doi-asserted-by": "crossref",
"first-page": "103104",
"journal-title": "EBioMedicine",
"key": "ref_49",
"volume": "61",
"year": "2020"
},
{
"DOI": "10.1038/s41418-021-00795-y",
"article-title": "Syncytia formation during SARS-CoV-2 lung infection: A disastrous unity to eliminate lymphocytes",
"author": "Lin",
"doi-asserted-by": "crossref",
"first-page": "2019",
"journal-title": "Cell Death Differ.",
"key": "ref_50",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1016/j.jmb.2021.167280",
"article-title": "The Mechanism and Consequences of SARS-CoV-2 Spike-Mediated Fusion and Syncytia Formation",
"author": "Rajah",
"doi-asserted-by": "crossref",
"first-page": "167280",
"journal-title": "J. Mol. Biol.",
"key": "ref_51",
"volume": "434",
"year": "2022"
},
{
"DOI": "10.1093/oxfordjournals.aje.a118408",
"article-title": "A simple method of estimating fifty per cent endpoints",
"author": "Reed",
"doi-asserted-by": "crossref",
"first-page": "493",
"journal-title": "Am. J. Epidemiol.",
"key": "ref_52",
"volume": "27",
"year": "1938"
},
{
"DOI": "10.3168/jds.2021-21247",
"article-title": "Evaluating the in vitro efficacy of bovine lactoferrin products against SARS-CoV-2 variants of concern",
"author": "Wotring",
"doi-asserted-by": "crossref",
"first-page": "2791",
"journal-title": "J. Dairy Sci.",
"key": "ref_53",
"volume": "105",
"year": "2022"
},
{
"DOI": "10.1371/journal.pbio.2005970",
"doi-asserted-by": "crossref",
"key": "ref_54",
"unstructured": "McQuin, C., Goodman, A., Chernyshev, V., Kamentsky, L., Cimini, B.A., Karhohs, K.W., Doan, M., Ding, L., Rafelski, S.M., and Thirstrup, D. (2018). CellProfiler 3.0: Next-generation image processing for biology. PLoS Biol., 16."
},
{
"DOI": "10.1145/1656274.1656280",
"article-title": "KNIME: The Konstanz information miner",
"author": "Berthold",
"doi-asserted-by": "crossref",
"first-page": "58",
"journal-title": "ACM SIGKDD Explor. Newsl.",
"key": "ref_55",
"volume": "11",
"year": "2009"
}
],
"reference-count": 55,
"references-count": 55,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2022.06.24.497526",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2076-393X/10/8/1284"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "In Vitro Evaluation and Mitigation of Niclosamide’s Liabilities as a COVID-19 Treatment",
"type": "journal-article",
"volume": "10"
}