Blockade of endolysosomal acidification suppresses TLR3-mediated pro-inflammatory signaling in airway epithelial cells
et al., Journal of Allergy and Clinical Immunology, doi:10.1016/j.jaci.2024.05.031, Jun 2024
56th treatment shown to reduce risk in
August 2025, now with p = 0.0069 from 7 studies.
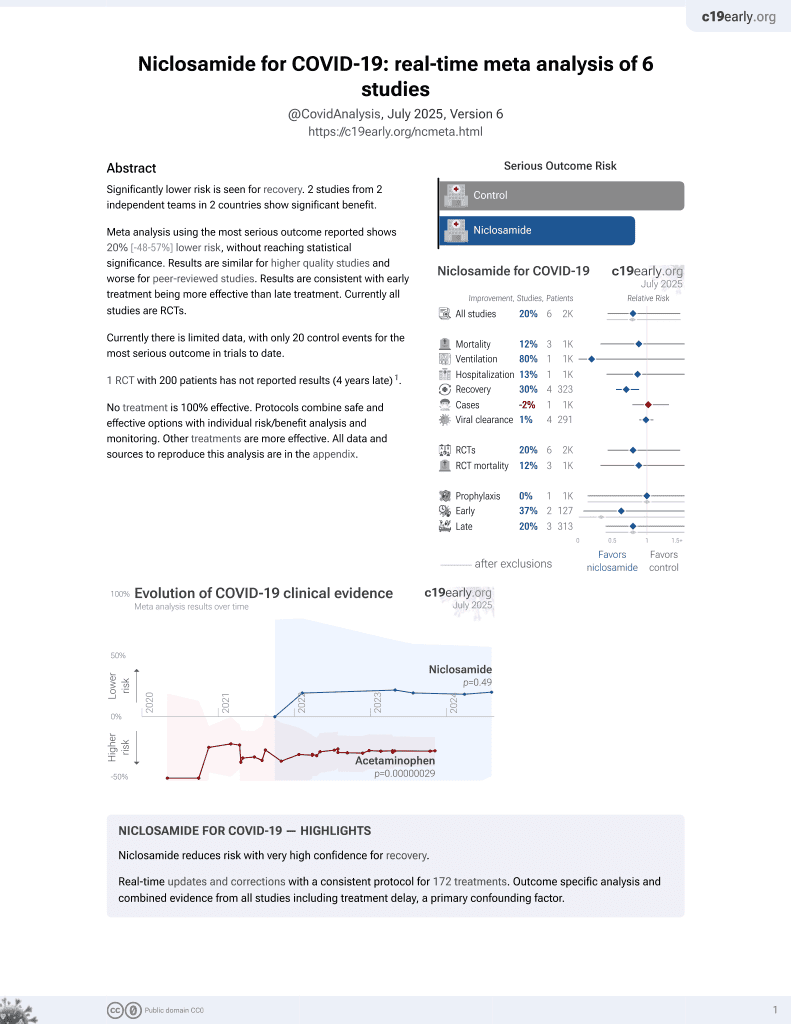
Lower risk for recovery.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
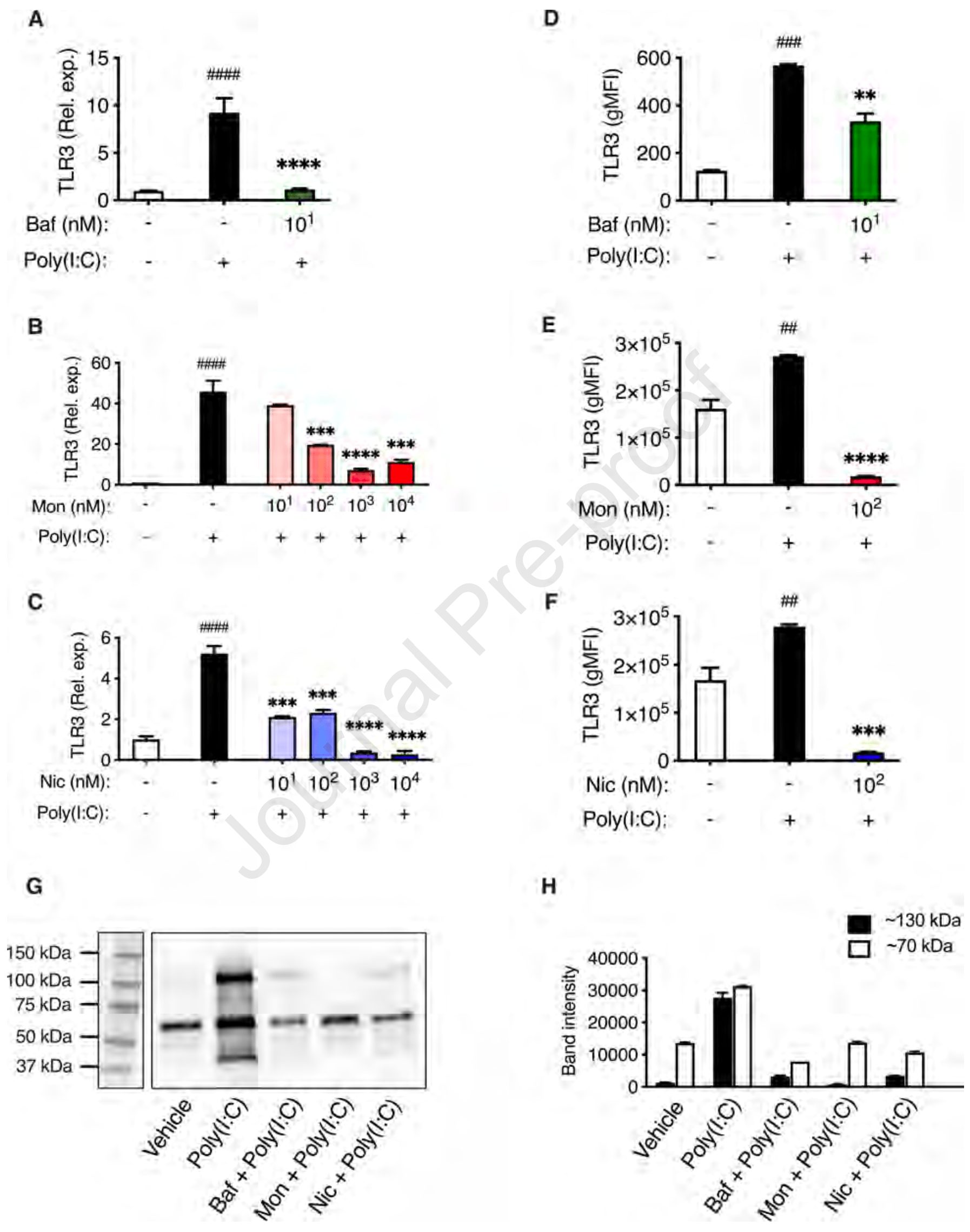
In vitro study showing that blocking endolysosomal acidification with bafilomycin A1, monensin, or niclosamide suppresses TLR3-mediated pro-inflammatory signaling in human small airway epithelial cells (HSAECs) stimulated with TLR3 agonists mimicking viral RNA. The inhibitors reduced expression of pro-inflammatory cytokines IL-6, TNF, IL-8 and IL-1β at both mRNA and protein levels. The findings suggest a potential therapeutic strategy to reduce excessive cytokine production in response to respiratory virus infection. HCQ is not used in this study but also blocks endolysosomal acidification and may have similar potential.
9 preclinical studies support the efficacy of niclosamide for COVID-19:
In silico studies predict inhibition of SARS-CoV-2 with niclosamide or metabolites via binding to the spikeA,1, MproB,1, RNA-dependent RNA polymeraseC,1, PLproD,1, nucleocapsidE,1, and helicaseF,1 proteins.
Niclosamide inhibits endolysosomal acidification and suppresses
TLR3-mediated pro-inflammatory signaling in human small airway
epithelial cells stimulated with TLR3 agonists mimicking viral RNA2, modulates host lipid metabolism and reduces
infectious SARS-CoV-2 virion production in Vero E6 cells4, reduces CD147 protein levels and inhibits
SARS-CoV-2-induced upregulation of CD147 in A549-ACE2 cells, including
the highly glycosylated form of CD147 which has been implicated in
COVID-19 disease progression and post-COVID-19 cardiac complications5, blocked the formation of syncytia mediated by
SARS-CoV-2 spike protein pseudovirus-producing cells6, may reduce inflammation, NLRP3 formation, and
caspase-1 activity9, may inhibit viral uncoating, replication, and
assembly via disruption of pH gradients and reduced ATP production in
host cells8, may counter immune evasion by reversing E-, ORF7a-, and ORF8-mediated down-regulation of MHC-I, preserving CD8⁺ T-cell recognition10, and shows strong synergy when combined with
ivermectin7.
Study covers niclosamide and HCQ.
1.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
2.
Pejler et al., Blockade of endolysosomal acidification suppresses TLR3-mediated pro-inflammatory signaling in airway epithelial cells, Journal of Allergy and Clinical Immunology, doi:10.1016/j.jaci.2024.05.031.
3.
Walia et al., SARS-CoV-2 virulence factor ORF3a blocks lysosome function by modulating TBC1D5-dependent Rab7 GTPase cycle, Nature Communications, doi:10.1038/s41467-024-46417-2.
4.
Garrett et al., Niclosamide as a chemical probe for analyzing SARS-CoV-2 modulation of host cell lipid metabolism, Frontiers in Microbiology, doi:10.3389/fmicb.2023.1251065.
5.
Yang et al., Repurposing Niclosamide as a Novel Anti-SARS-CoV-2 Drug by Restricting Entry Protein CD147, Biomedicines, doi:10.3390/biomedicines11072019.
6.
Sheng et al., A pseudovirus-based method to dynamically mimic SARS-CoV-2-associated cell-to-cell fusion and transmission, Acta Biochimica et Biophysica Sinica, doi:10.3724/abbs.2023129.
7.
Jitobaom et al., Synergistic anti-SARS-CoV-2 activity of repurposed anti-parasitic drug combinations, BMC Pharmacology and Toxicology, doi:10.1186/s40360-022-00580-8.
8.
Needham, D., The pH Dependence of Niclosamide Solubility, Dissolution, and Morphology: Motivation for Potentially Universal Mucin-Penetrating Nasal and Throat Sprays for COVID19, its Variants and other Viral Infections, Pharmaceutical Research, doi:10.1007/s11095-021-03112-x.
a.
The trimeric spike (S) protein is a glycoprotein that mediates viral entry by binding to the host ACE2 receptor, is critical for SARS-CoV-2's ability to infect host cells, and is a target of neutralizing antibodies. Inhibition of the spike protein prevents viral attachment, halting infection at the earliest stage.
b.
The main protease or Mpro, also known as 3CLpro or nsp5, is a cysteine protease that cleaves viral polyproteins into functional units needed for replication. Inhibiting Mpro disrupts the SARS-CoV-2 lifecycle within the host cell, preventing the creation of new copies.
c.
RNA-dependent RNA polymerase (RdRp), also called nsp12, is the core enzyme of the viral replicase-transcriptase complex that copies the positive-sense viral RNA genome into negative-sense templates for progeny RNA synthesis. Inhibiting RdRp blocks viral genome replication and transcription.
d.
The papain-like protease (PLpro) has multiple functions including cleaving viral polyproteins and suppressing the host immune response by deubiquitination and deISGylation of host proteins. Inhibiting PLpro may block viral replication and help restore normal immune responses.
e.
The nucleocapsid (N) protein binds and encapsulates the viral genome by coating the viral RNA. N enables formation and release of infectious virions and plays additional roles in viral replication and pathogenesis. N is also an immunodominant antigen used in diagnostic assays.
f.
The helicase, or nsp13, protein unwinds the double-stranded viral RNA, a crucial step in replication and transcription. Inhibition may prevent viral genome replication and the creation of new virus components.
Pejler et al., 19 Jun 2024, multiple countries, peer-reviewed, 4 authors.
Contact: aida.paivandy@uu.se.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Blockade of endolysosomal acidification suppresses TLR3-mediated pro-inflammatory signaling in airway epithelial cells
Journal of Allergy and Clinical Immunology, doi:10.1016/j.jaci.2024.05.031
Background: Endolysosomal compartments are acidic and contain low pH-dependent proteases, and these conditions are exploited by respiratory viruses, such as SARS-CoV-2 and influenza virus, for escaping into the cytosol. Moreover, endolysosomes contain various pattern recognition receptors (PRRs), which respond to virus-derived pathogen-associated molecular patterns (PAMPs) by production of pro-inflammatory cytokines/chemokines. However, excessive pro-inflammatory responses can lead to a potentially lethal cytokine storm. Objectives: Here we investigated the endosomal PRR expression profile in primary human small airway epithelial cells (HSAECs), and whether blockade of endolysosomal acidification affects their cytokine/chemokine production after challenge with virus-derived stimulants. Methods: HSAECs were exposed to stimulants mimicking virus-derived PAMPs, either in the absence or presence of compounds causing blockade of endolysosomal acidification, followed by measurement of cytokine expression and release. Results: We show that toll-like receptor 3 (TLR3) is the major endosomal PRR expressed by HSAECs, and that TLR3 expression is strongly induced by TLR3 agonists, but not by a range of other PRR agonists. We also demonstrate that TLR3 engagement with its agonists elicits a robust pro-inflammatory cytokine/chemokine response, which is profoundly suppressed through blockade of endolysosomal acidification, by bafilomycin A1, monensin, or niclosamide. Using TLR3 reporter cells, it was confirmed that TLR3 signaling is strongly induced by Poly(I:C) and that blockade of endolysosomal acidification efficiently blocked TLR3 signaling. Finally, we show that blockade of endolysosomal acidification causes a reduction in the levels of TLR3 mRNA and protein.
Conclusion: These findings show that blockade of endolysosomal acidification suppresses TLR3-dependent cytokine and chemokine production in HSAECs. Clinical implication: These findings may be exploited for therapeutic strategies aiming to ameliorate the cytokine storm in response to respiratory virus infection. Capsule summary. This study shows that blockade of endolysosomal acidification in human airway epithelial cells causes reduced TLR3 signaling and reduced output of proinflammatory cytokines and chemokines.
References
Bird, Trapani, Villadangos, Endolysosomal proteases and their inhibitors in immunity, Nat Rev Immunol
Blasius, Beutler, Intracellular toll-like receptors, Immunity
Bortolotti, Gentili, Rizzo, Schiuma, Beltrami et al., TLR3 and TLR7 RNA Sensor Activation during SARS-CoV-2 Infection, Microorganisms
Botos, Liu, Wang, Segal, Davies, The toll-like receptor 3:dsRNA signaling complex, Biochim Biophys Acta
Brunaugh, Seo, Warnken, Ding, Seo et al., Development and evaluation of inhalable composite niclosamide-lysozyme particles: A broad-spectrum, patient-adaptable treatment for coronavirus infections and sequalae, PLoS One
Cairns, Boorgu, Levin, Kaplan, Niclosamide rescues microcephaly in a humanized in vivo model of Zika infection using human induced neural stem cells, Biol Open
Cario, Podolsky, Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease, Infect Immun
Dai, Wang, Wang, Gao, Wang et al., Toll-Like Receptor Signaling in Severe Acute Respiratory Syndrome Coronavirus 2-Induced Innate Immune Responses and the Potential Application Value of Toll-Like Receptor Immunomodulators in Patients With Coronavirus Disease 2019, Front Microbiol
Dauletbaev, Cammisano, Herscovitch, Lands, Stimulation of the RIG-I/MAVS Pathway by Polyinosinic:Polycytidylic Acid Upregulates IFN-beta in Airway Epithelial Cells with Minimal Costimulation of IL-8, J Immunol
Ditzel, Schwartz, Worm cure without tears. The effect of niclosamide on taeniasis saginata in man, Acta Med Scand
Edinger, Pohl, Yanguez, Stertz, Cathepsin W Is Required for Escape of Influenza A Virus from Late Endosomes, mBio
Ewald, Engel, Lee, Wang, Bogyo et al., Nucleic acid recognition by Toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase, J Exp Med
Forgac, Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology, Nat Rev Mol Cell Biol
Garcia-Cattaneo, Gobert, Muller, Toscano, Flores et al., Cleavage of Toll-like receptor 3 by cathepsins B and H is essential for signaling, Proc Natl Acad Sci U S A
Gassen, Niemeyer, Muth, Corman, Martinelli et al., SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection, Nat Commun
Goffic, Pothlichet, Vitour, Fujita, Meurs et al., Cutting Edge: Influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells, J Immunol
Herold, Jurinovic, Arnreich, Lipworth, Hellmuth et al., Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19, J Allergy Clin Immunol
Herrera-Rodriguez, Signorazzi, Holtrop, De Vries-Idema, Huckriede, Inactivated or damaged? Comparing the effect of inactivation methods on influenza virions to optimize vaccine production, Vaccine
Investigators, Gordon, Mouncey, Al-Beidh, Rowan et al., Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19, N Engl J Med
Ishii, Funami, Tatematsu, Seya, Matsumoto, Endosomal localization of TLR8 confers distinctive proteolytic processing on human myeloid cells, J Immunol
J O U R N A L P R E, None
J O U R N A L P R E, None
Jackson, Farzan, Chen, Choe, Mechanisms of SARS-CoV-2 entry into cells, Nat Rev Mol Cell Biol
Jarver, Dondalska, Poux, Sandberg, Bergenstrahle et al., Single-Stranded Nucleic Acids Regulate TLR3/4/7 Activation through Interference with Clathrin-Mediated Endocytosis, Sci Rep
Jung, Nam, Oh, Jun, Ro et al., Neutralization of Acidic Intracellular Vesicles by Niclosamide Inhibits Multiple Steps of the Dengue Virus Life Cycle In Vitro, Sci Rep
Jurgeit, Mcdowell, Moese, Meldrum, Schwendener et al., Niclosamide is a proton carrier and targets acidic endosomes with broad antiviral effects, PLoS Pathog
Kao, Huangfu, Tsai, Ho, Jhan et al., The antiparasitic drug niclosamide inhibits dengue virus infection by interfering with endosomal acidification independent of mTOR, PLoS Negl Trop Dis
Kawai, Akira, Toll-like receptor and RIG-I-like receptor signaling, Ann N Y Acad Sci
Kawasaki, Kawai, Toll-like receptor signaling pathways, Front Immunol
Kircheis, Haasbach, Lueftenegger, Heyken, Ocker et al., NF-kappaB Pathway as a Potential Target for Treatment of Critical Stage COVID-19 Patients, Front Immunol
Ko, Cha, Lee, Bae, Ham et al., A novel defined TLR3 agonist as an effective vaccine adjuvant, Front Immunol
Leonard, Ghirlando, Askins, Bell, Margulies et al., The TLR3 signaling complex forms by cooperative receptor dimerization, Proc Natl Acad Sci U S A
Maccarana, Liu, Lampinen, Rollman, Adner et al., Monensin induces selective mast cell apoptosis through a secretory granule-mediated pathway, Allergy
Malik, Zhou, Innate Immune Sensing of Influenza A Virus, Viruses
Matsumoto, Oshiumi, Seya, Antiviral responses induced by the TLR3 pathway, Rev Med Virol
Misinzo, Delputte, Nauwynck, Inhibition of endosome-lysosome system acidification enhances porcine circovirus 2 infection of porcine epithelial cells, J Virol
Mollenhauer, Morre, Rowe, Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity, Biochim Biophys Acta
Murer, Petkidis, Vallet, Vignuzzi, Greber, Chemical Evolution of Rhinovirus Identifies Capsid-Destabilizing Mutations Driving Low-pH-Independent Genome Uncoating, J Virol
Nakamura, Funami, Komori, Yokoyama, Aiba et al., Increased expression of Toll-like receptor 3 in intrahepatic biliary epithelial cells at sites of ductular reaction in diseased livers, Hepatol Int
Naumann, Wehner, Schwarze, Petzold, Schmitz et al., Activation of dendritic cells by the novel Toll-like receptor 3 agonist RGC100, Clin Dev Immunol
Niimi, Asano, Shiraishi, Nakajima, Wakaki et al., TLR3-mediated synthesis and release of eotaxin-1/CCL11 from human bronchial smooth muscle cells stimulated with double-stranded RNA, J Immunol
Prabhakara, Godbole, Sil, Jahnavi, Gulzar et al., Strategies to target SARS-CoV-2 entry and infection using dual mechanisms of inhibition by acidification inhibitors, PLoS Pathog
Qi, Singh, Kao, Proteolytic processing regulates Toll-like receptor 3 stability and endosomal localization, J Biol Chem
Ritter, Mennerich, Weith, Seither, Characterization of Toll-like receptors in primary lung epithelial cells: strong impact of the TLR3 ligand poly(I:C) on the regulation of Toll-like receptors, adaptor proteins and inflammatory response, J Inflamm (Lond)
Sha, Truong-Tran, Plitt, Beck, Schleimer, Activation of airway epithelial cells by toll-like receptor agonists, Am J Respir Cell Mol Biol
Shang, Zhuang, Zhang, Li, Zhu et al., Inhibitors of endosomal acidification suppress SARS-CoV-2 replication and relieve viral pneumonia in hACE2 transgenic mice, Virol J
Singh, Weiss, Goodman, Fisk, Kulkarni et al., Niclosamide-A promising treatment for COVID-19, Br J Pharmacol
Suzuki, Yamaya, Sekizawa, Hosoda, Yamada et al., Bafilomycin A(1) inhibits rhinovirus infection in human airway epithelium: effects on endosome and ICAM-1, Am J Physiol Lung Cell Mol Physiol
Toscano, Estornes, Virard, Garcia-Cattaneo, Pierrot et al., Cleaved/associated TLR3 represents the primary form of the signaling receptor, J Immunol
Valle, Kim-Schulze, Huang, Beckmann, Nirenberg et al., An inflammatory cytokine signature predicts COVID-19 severity and survival, Nat Med
Weiss, Touret, Baronti, Gilles, Hoen et al., Niclosamide shows strong antiviral activity in a human airway model of SARS-CoV-2 infection and a conserved potency against the Alpha (B.1.1.7), Beta (B.1.351) and Delta variant (B.1.617.2), PLoS One
Wen, Kuo, Jan, Liang, Wang et al., Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus, J Med Chem
Wu, Jan, Chen, Hsieh, Hwang et al., Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide, Antimicrob Agents Chemother
Yamaya, Deng, Kikuchi, Sugawara, Saito et al., The proton ATPase inhibitor bafilomycin A1 reduces the release of rhinovirus C and cytokines from primary cultures of human nasal epithelial cells, Virus Res
Yang, Peng, Hsu, Lee, Wu et al., Repurposing old drugs as antiviral agents for coronaviruses, Biomed J
Yeganeh, Ghavami, Kroeker, Mahood, Stelmack et al., Suppression of influenza A virus replication in human lung epithelial cells by noncytotoxic concentrations bafilomycin A1, Am J Physiol Lung Cell Mol Physiol
Zhao, Lampinen, Rollman, Sommerhoff, Paivandy et al., Mast cell chymase affects the functional properties of primary human airway fibroblasts: Implications for asthma, J Allergy Clin Immunol
Zhao, Meng, Peng, Lam, Zhang et al., Fusion-inhibition peptide broadly inhibits influenza virus and SARS-CoV-2, including Delta and Omicron variants, Emerg Microbes Infect
Zhao, Sommerhoff, Paivandy, Pejler, Mast cell chymase regulates extracellular matrix remodeling-related events in primary human small airway epithelial cells, J Allergy Clin Immunol
Zhao, To, Sze, Yung, Bian et al., A broad-spectrum virus-and host-targeting peptide against respiratory viruses including influenza virus and SARS-CoV-2, Nat Commun
DOI record:
{
"DOI": "10.1016/j.jaci.2024.05.031",
"ISSN": [
"0091-6749"
],
"URL": "http://dx.doi.org/10.1016/j.jaci.2024.05.031",
"alternative-id": [
"S0091674924006079"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Blockade of endolysosomal acidification suppresses TLR3-mediated pro-inflammatory signaling in airway epithelial cells"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Allergy and Clinical Immunology"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jaci.2024.05.031"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 Published by Elsevier Inc. on behalf of the American Academy of Allergy, Asthma & Immunology."
}
],
"author": [
{
"affiliation": [],
"family": "Pejler",
"given": "Gunnar",
"sequence": "first"
},
{
"affiliation": [],
"family": "Zhao",
"given": "Xinran O.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fagerström",
"given": "Ella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paivandy",
"given": "Aida",
"sequence": "additional"
}
],
"container-title": "Journal of Allergy and Clinical Immunology",
"container-title-short": "Journal of Allergy and Clinical Immunology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.com",
"jacionline.org",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
6,
19
]
],
"date-time": "2024-06-19T23:16:27Z",
"timestamp": 1718838987000
},
"deposited": {
"date-parts": [
[
2024,
6,
21
]
],
"date-time": "2024-06-21T01:22:28Z",
"timestamp": 1718932948000
},
"funder": [
{
"DOI": "10.13039/100007436",
"doi-asserted-by": "publisher",
"name": "Erling Persson Family Foundation"
},
{
"DOI": "10.13039/501100002794",
"doi-asserted-by": "publisher",
"name": "Cancerfonden"
},
{
"DOI": "10.13039/501100004359",
"doi-asserted-by": "publisher",
"name": "Vetenskapsradet"
},
{
"DOI": "10.13039/501100004063",
"doi-asserted-by": "publisher",
"name": "Knut Och Alice Wallenbergs Stiftelse"
},
{
"DOI": "10.13039/501100003793",
"doi-asserted-by": "publisher",
"name": "Hjärt-lungfonden"
}
],
"indexed": {
"date-parts": [
[
2024,
6,
22
]
],
"date-time": "2024-06-22T00:25:36Z",
"timestamp": 1719015936169
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
6
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
6,
1
]
],
"date-time": "2024-06-01T00:00:00Z",
"timestamp": 1717200000000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
6,
1
]
],
"date-time": "2024-06-01T00:00:00Z",
"timestamp": 1717200000000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 16,
"start": {
"date-parts": [
[
2024,
6,
17
]
],
"date-time": "2024-06-17T00:00:00Z",
"timestamp": 1718582400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0091674924006079?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0091674924006079?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
6
]
]
},
"published-print": {
"date-parts": [
[
2024,
6
]
]
},
"publisher": "Elsevier BV",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0091674924006079"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Blockade of endolysosomal acidification suppresses TLR3-mediated pro-inflammatory signaling in airway epithelial cells",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}