The pH Dependence of Niclosamide Solubility, Dissolution, and Morphology: Motivation for Potentially Universal Mucin-Penetrating Nasal and Throat Sprays for COVID19, its Variants and other Viral Infections
, D., Pharmaceutical Research, doi:10.1007/s11095-021-03112-x, Dec 2021
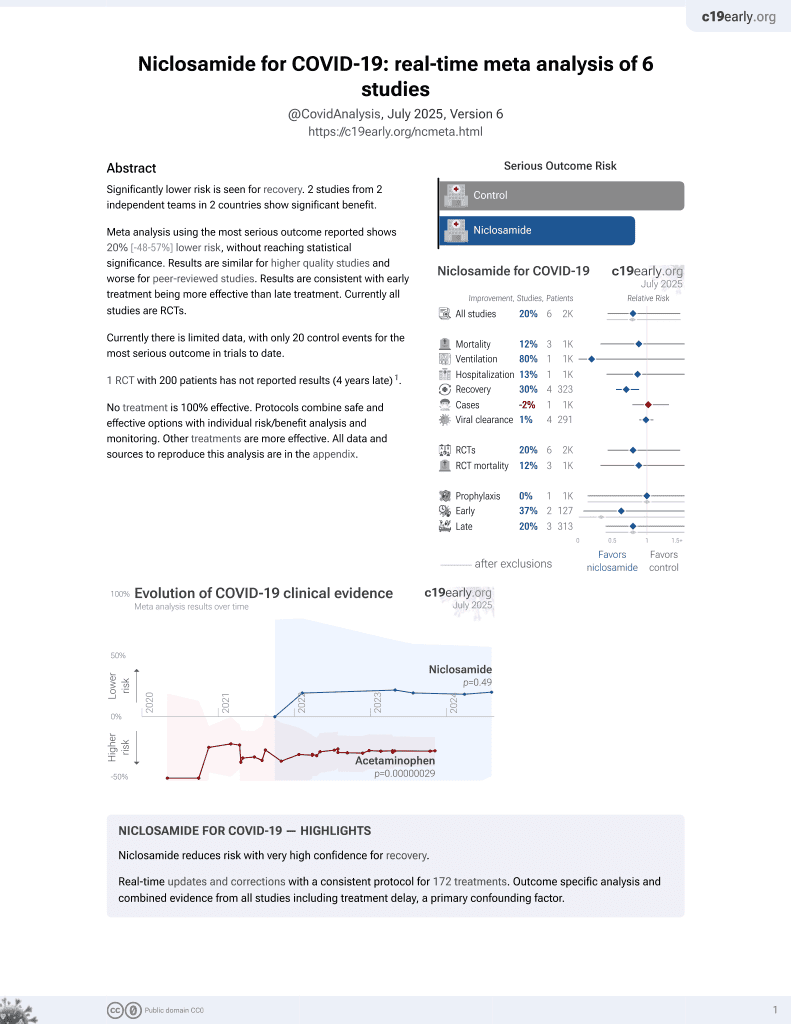
56th treatment shown to reduce risk in
August 2025, now with p = 0.0069 from 7 studies.
Lower risk for recovery.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
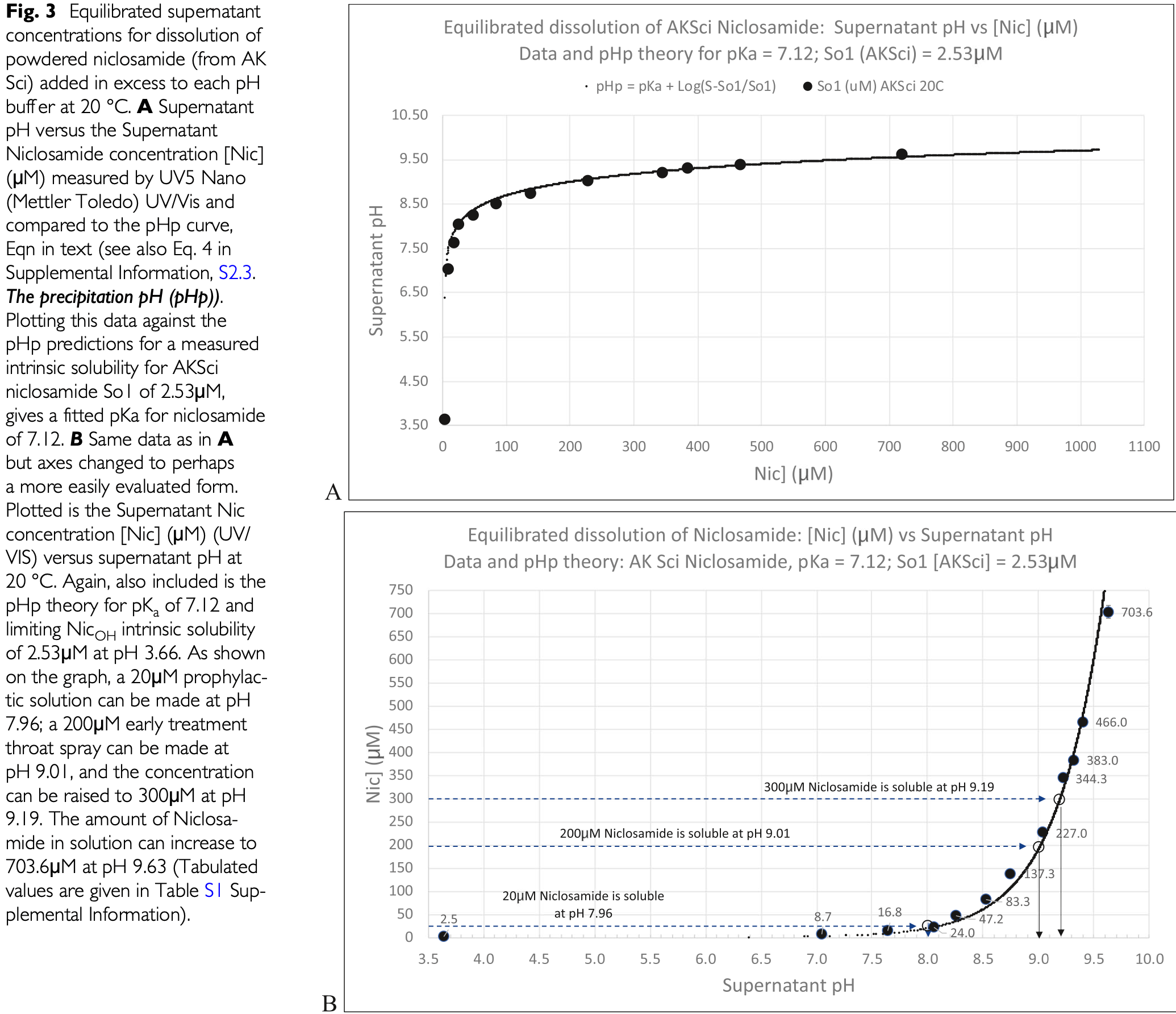
In vitro study showing that niclosamide has potential as a prophylactic nasal spray and early treatment throat spray for COVID-19 and other respiratory viral infections. Authors find that niclosamide's solubility and dissolution rate are pH-dependent, with concentrations increasing from 2.53μM at pH 3.66 to 704μM at pH 9.63. A 20μM prophylactic nasal spray can be formulated at pH 7.96, while a 300μM early treatment throat spray can be formulated at pH 9.19. These concentrations are 10-150 times higher than the 100% inhibitory concentration of 1-3μM reported for SARS-CoV-2 infected Vero 6 and Calu-3 cells. Niclosamide's broad antiviral activity is attributed to its ability to disrupt pH gradients and reduce ATP production in host cells, inhibiting viral uncoating, replication, and assembly. Niclosamide inhibits the following stages of viral infection: uncoating - preventing viral RNA release from the endosome; replication - reducing ATP available for viral transcription and translation; and assembly - interfering with capsid assembly in the Golgi, promoting secretion of non-competent virions.
9 preclinical studies support the efficacy of niclosamide for COVID-19:
In silico studies predict inhibition of SARS-CoV-2 with niclosamide or metabolites via binding to the spikeA,1, MproB,1, RNA-dependent RNA polymeraseC,1, PLproD,1, nucleocapsidE,1, and helicaseF,1 proteins.
Niclosamide inhibits endolysosomal acidification and suppresses
TLR3-mediated pro-inflammatory signaling in human small airway
epithelial cells stimulated with TLR3 agonists mimicking viral RNA2, modulates host lipid metabolism and reduces
infectious SARS-CoV-2 virion production in Vero E6 cells4, reduces CD147 protein levels and inhibits
SARS-CoV-2-induced upregulation of CD147 in A549-ACE2 cells, including
the highly glycosylated form of CD147 which has been implicated in
COVID-19 disease progression and post-COVID-19 cardiac complications5, blocked the formation of syncytia mediated by
SARS-CoV-2 spike protein pseudovirus-producing cells6, may reduce inflammation, NLRP3 formation, and
caspase-1 activity9, may inhibit viral uncoating, replication, and
assembly via disruption of pH gradients and reduced ATP production in
host cells8, may counter immune evasion by reversing E-, ORF7a-, and ORF8-mediated down-regulation of MHC-I, preserving CD8⁺ T-cell recognition10, and shows strong synergy when combined with
ivermectin7.
1.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
2.
Pejler et al., Blockade of endolysosomal acidification suppresses TLR3-mediated pro-inflammatory signaling in airway epithelial cells, Journal of Allergy and Clinical Immunology, doi:10.1016/j.jaci.2024.05.031.
3.
Walia et al., SARS-CoV-2 virulence factor ORF3a blocks lysosome function by modulating TBC1D5-dependent Rab7 GTPase cycle, Nature Communications, doi:10.1038/s41467-024-46417-2.
4.
Garrett et al., Niclosamide as a chemical probe for analyzing SARS-CoV-2 modulation of host cell lipid metabolism, Frontiers in Microbiology, doi:10.3389/fmicb.2023.1251065.
5.
Yang et al., Repurposing Niclosamide as a Novel Anti-SARS-CoV-2 Drug by Restricting Entry Protein CD147, Biomedicines, doi:10.3390/biomedicines11072019.
6.
Sheng et al., A pseudovirus-based method to dynamically mimic SARS-CoV-2-associated cell-to-cell fusion and transmission, Acta Biochimica et Biophysica Sinica, doi:10.3724/abbs.2023129.
7.
Jitobaom et al., Synergistic anti-SARS-CoV-2 activity of repurposed anti-parasitic drug combinations, BMC Pharmacology and Toxicology, doi:10.1186/s40360-022-00580-8.
8.
Needham, D., The pH Dependence of Niclosamide Solubility, Dissolution, and Morphology: Motivation for Potentially Universal Mucin-Penetrating Nasal and Throat Sprays for COVID19, its Variants and other Viral Infections, Pharmaceutical Research, doi:10.1007/s11095-021-03112-x.
a.
The trimeric spike (S) protein is a glycoprotein that mediates viral entry by binding to the host ACE2 receptor, is critical for SARS-CoV-2's ability to infect host cells, and is a target of neutralizing antibodies. Inhibition of the spike protein prevents viral attachment, halting infection at the earliest stage.
b.
The main protease or Mpro, also known as 3CLpro or nsp5, is a cysteine protease that cleaves viral polyproteins into functional units needed for replication. Inhibiting Mpro disrupts the SARS-CoV-2 lifecycle within the host cell, preventing the creation of new copies.
c.
RNA-dependent RNA polymerase (RdRp), also called nsp12, is the core enzyme of the viral replicase-transcriptase complex that copies the positive-sense viral RNA genome into negative-sense templates for progeny RNA synthesis. Inhibiting RdRp blocks viral genome replication and transcription.
d.
The papain-like protease (PLpro) has multiple functions including cleaving viral polyproteins and suppressing the host immune response by deubiquitination and deISGylation of host proteins. Inhibiting PLpro may block viral replication and help restore normal immune responses.
e.
The nucleocapsid (N) protein binds and encapsulates the viral genome by coating the viral RNA. N enables formation and release of infectious virions and plays additional roles in viral replication and pathogenesis. N is also an immunodominant antigen used in diagnostic assays.
f.
The helicase, or nsp13, protein unwinds the double-stranded viral RNA, a crucial step in replication and transcription. Inhibition may prevent viral genome replication and the creation of new virus components.
Needham et al., 28 Dec 2021, peer-reviewed, 1 author.
Contact: d.needham@duke.edu.
The pH Dependence of Niclosamide Solubility, Dissolution, and Morphology: Motivation for Potentially Universal Mucin-Penetrating Nasal and Throat Sprays for COVID19, its Variants and other Viral Infections
Pharmaceutical Research, doi:10.1007/s11095-021-03112-x
63. However, the "Sigma-polymorph" equilibrated to much lower final supernatant concentrations, reflective of more stable polymorphs at each pH. Similarly, when precipitated from supersaturated solution, or as cosolvates, niclosamide also equilibrated to lower final supernatant concentrations. Polymorph equilibration though was avoided by using a solvent-exchange technique to make the solutions. Conclusions Given niclosamide's activity as a host cell modulator, optimized niclosamide solutions could represent universal prophylactic nasal and early treatment throat sprays against COVID19, its more contagious variants, and other respiratory viral infections. They are the simplest and potentially most effective formulations from both an efficacy standpoint as well as manufacturing and distribution, (no cold chain). They now just need testing.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1007/ s11095-021-03112-x.
Declarations Conflict of Interest Statement Duke University has assigned the rights for the provisional patent applications to David Needham and his entity, Needham Material Science LLC. Author contributions for all authors David Needham was the sole contributor and sole author.
Data Availability Statement The datasets generated during and/ or analyzed during the current study are available from the corresponding author on reasonable request. Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Abrams, Seftel, Heinz, The treatment of human tapeworm infections with 'Yomesan, S Afr Med J
Baldwin, Scott, Mometasone furoate: a review of its intranasal use in allergic rhinitis, Drugs
Bayer, YOMESAN® tablets
Boegh, Nielsen, Mucus as a barrier to drug delivery -understanding and mimicking the barrier properties, Basic Clin Pharmacol Toxicol
Braga, Ali, Secco, Chiavacci, Neves et al., Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia, Nature
Brunaugh, Seo, Warnken, Ding, Seo et al., Development and evaluation of inhalable composite niclosamide-lysozyme particles: A broad-spectrum, patientadaptable treatment for coronavirus infections and sequalae, PloS One
Burley, Needham, Characterization of Niclosamide (XRD, FT-IR/Raman, DSC) from various suppliers and as precipitates and cosolvates confirms polymorphic forms as a function of pH
Cabrita, Benedetto, Schreiber, Kunzelmann, Niclosamide repurposed for the treatment of inflammatory airway disease, JCI Insight
Caira, Van Tonder, Villiers, Lötter, Diverse Modes of Solvent Inclusion in Crystalline Pseudopolymorphs of the Anthelmintic Drug Niclosamide, J Inclusion Phenom Mol Recognit Chem
Cdc, SARS-CoV-2 Variant Classifications and Definitions
Chang, Li, Hsu, Chang, Lo, Increased ATP generation in the host cell is required for efficient vaccinia virus production, J Biomed Sci
Chen, Mook, Premont, Wang, Niclosamide: Beyond an antihelminthic drug, Cell Signal
Cheng, Morales, Zhang, Mito, Tsin, Niclosamide induces protein ubiquitination and inhibits multiple prosurvival signaling pathways in the human glioblastoma U-87 MG cell line, PloS One
Conaway, Conaway, ATP activates transcription initiation from promoters by RNA polymerase II in a reversible step prior to RNA synthesis, J Biol Chem
Daewoong-Pharmaceutical, Daewoong Pharmaceutical Partners with Tufts Medical Center for Phase 2 Clinical Trial with Niclosamide
De Villiers, Mahlatji, Van Tonder, Malan, Lötter et al., Comparison of the physical and chemical stability of niclosamide crystal forms in aqueous versus nonaqueous suspensions, Drug Dev Ind Pharm
Duncan, Needham, Microdroplet dissolution into a second-phase solvent using a micropipet technique: Test of 1 3 the Epstein-Plesset model for an aniline-water system, Langmuir
Duncan, Needham, Test of the Epstein-Plesset model for gas microparticle dissolution in aqueous media: Effect of surface tension and gas undersaturation in solution, Langmuir
Epstein, Plesset, On the Stability of Gas Bubbles in Liquid-Gas Solutions, J Chem Phys
Goulding, and-disea se/ virus-repli cation
Halász, Gyüre, Jánosi, Vortex flow generated by a magnetic stirrer, Am J Phys
Hernandez, Nearly 94,000 Kids Got COVID-19 Last Week
Hou, Okuda, Edwards, Martinez, Asakura et al., SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract, Cell
Huang, Chen, Yang, Guan, Liu et al., SARS-CoV-2 Viral Load in Clinical Samples from Critically Ill Patients, Am J Respir Crit Care Med
Imperi, Massai, Pillai, Longo, Zennaro et al., New life for an old drug: the anthelmintic drug niclosamide inhibits Pseudomonas aeruginosa quorum sensing, Antimicrob Agents Chemother
Jara, Warnken, Sahakijpijarn, Moon, Maier et al., Niclosamide Inhalation Powder Made by Thin-Film Freezing: Pharmacokinetic and Toxicology Studies in Rats and Hamsters, bioRxiv
Jeon, Ko, Lee, Choi, Byun et al., Identification of Antiviral Drug Candidates against SARS-CoV-2 from FDA-Approved Drugs, Antimicrob Agents Chemother
Jeon, Ko, Lee, Choi, Byun et al., Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs, bioRxiv
Jones, The nose and paranasal sinuses physiology and anatomy, Adv Drug Deliv Rev
Jung, Nam, Oh, Jun, Ro et al., Neutralization of Acidic Intracellular Vesicles by Niclosamide Inhibits Multiple Steps of the Dengue Virus Life Cycle In Vitro, Sci Rep
Jurgeit, Mcdowell, Moese, Meldrum, Schwendener et al., Niclosamide Is a Proton Carrier and Targets Acidic Endosomes with Broad Antiviral Effects, PLOS Pathogens
Kadri, Lambourne, Mehellou, Niclosamide, a Drug with Many (Re)purposes, ChemMedChem
Khanim, Merrick, Giles, Jankute, Jackson et al., Redeployment-based drug screening identifies the anti-helminthic niclosamide as anti-myeloma therapy that also reduces free light chain production, Blood Cancer J
Kim, Bear, Needham, Lee, Effect of Niclosamide on cytotoxicity and cell viability in small airway epithelial cells in preparation
Kim, Costello, Duncan, Needham, Mechanical properties and microstructure of polycrystalline phospholipid monolayer shells: Novel solid microparticles, Langmuir
Kim, Klibanov, Evans, Needham, Viscoelastic properties of phospholipid monolayer shells on air microbubbles, Biophys J
Ko, Jeon, Ryu, Kim, Comparative analysis of antiviral efficacy of FDA-approved drugs against SARS-CoV-2 in 1 3 human lung cells: Nafamostat is the most potent antiviral drug candidate, bioRxiv
Laise, Bosker, Sun, Shen, Douglass et al., The Host Cell ViroCheckpoint: Identification and Pharmacologic Targeting of Novel Mechanistic Determinants of Coronavirus-Mediated Hijacked Cell States. bioRxiv : the preprint server for biology
Leal, Smyth, Ghosh, Physicochemical properties of mucus and their impact on transmucosal drug delivery, Int J Pharm
Liu, Lou, Zhu, Nadiminty, Schwartz et al., Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer, Clin Cancer Res
Llinàs, Box, Burley, Glen, Goodman, A new method for the reproducible generation of polymorphs: two forms of sulindac with very different solubilities, J Appl Crystallogr
Mccollum, Conde-Vancells, Hans, Vazquez-Chantada, Kleinstreuer et al., Identification of vascular disruptor compounds by analysis in zebrafish embryos and mouse embryonic endothelial cells, Reprod Toxicol
Mcconville, Hubert, Remucal, Direct Photolysis Rates and Transformation Pathways of the Lampricides TFM and Niclosamide in Simulated Sunlight, Environ Sci Technol
Merck, Prescribing information Nasonex
Miner, Labitzke, Liu, Wang, Henckels et al., Drug Repurposing: The Anthelmintics Niclosamide and Nitazoxanide Are Potent TMEM16A Antagonists That Fully Bronchodilate Airways, Front Pharmacol
Mook, Zhao, Abstract 5491: Interrogating the mechanism of Wnt pathway inhibition by niclosamide, Can Res
Mostafa, Kandeil, Elshaier, Kutkat, Moatasim et al., FDA-Approved Drugs with Potent In Vitro Antiviral Activity against Severe Acute Respiratory Syndrome Coronavirus 2, Pharmaceuticals
Nagy, Taking over Cellular Energy-Metabolism for TBSV Replication: The High ATP Requirement of an RNA Virus within the Viral Replication Organelle, Viruses
Needham, Arslanagic, Glud, Hervella, Karimi et al., Bottom up design of nanoparticles for anti-cancer diapeutics, J Drug Target
Needham, University, Current Provisional Patent Portfolio USPO: a) DU7305PROV
Needham, White, Paper, Why Niclosamide? A Basis for New Nasal and Throat Spray Solution-Formulation for COVID19 and Other Viral Infections
Ourworldindata, Share of people vaccinated against COVID-19
Paul, Samatha, Manasa, Munemma, Supriya, Modeling the oral cavity with mucoadhesive drug delivery systems -a potential alternative to conventional therapy, Int J Pharm Sci Drug Res
Prabhakara, Godbole, Sil, Jahnavi, Van Zanten et al., Niclosamide inhibits SARS-CoV2 entry by blocking internalization through pH-dependent CLIC/ GEEC endocytic pathway, bioRxiv
Reddy, Kerr, Spasojevic, Tovmasyan, Hsu et al., Preclinical Testing of a Novel Niclosamide Stearate Prodrug Therapeutic (NSPT) shows efficacy against Osteosarcoma, Mol Cancer Ther
Schweizer, Haugk, Mckiernan, Gulati, Cheng et al., A phase I study of niclosamide in combination with enzalutamide in men with castration-resistant prostate cancer, PLOS ONE
Shah, FLONASE (fluticasone propionate) nasal spray. Prescribing information
Shang, Wan, Luo, Ye, Geng et al., Cell entry mechanisms of SARS-CoV-2, Proc Natl Acad Sci U S A
Su, Needham, Mass transfer in the dissolution of a multicomponent liquid droplet in an immiscible liquid environment, Langmuir
Tran, Labanowski, Gallard, Adsorption and transformation of the anthelmintic drug niclosamide by manganese oxide, Chemosphere
Van Tonder, Mahlatji, Malan, Liebenberg, Caira et al., Preparation and physicochemical characterization of 5 niclosamide solvates and 1 hemisolvate, AAPS PharmSciTech
Van Tonder, Maleka, Liebenberg, Song, Wurster et al., Preparation and physicochemical properties of niclosamide anhydrate and two monohydrates, Int J Pharm
Washington, Steele, Jackson, Bush, Mason et al., Determination of baseline human nasal pH and the effect of intranasally administered buffers, Int J Pharm
Xu, Shi, Li, Zhou, Broad Spectrum Antiviral Agent Niclosamide and Its Therapeutic Potential, ACS Infect Dis
Zimmerman, Saint André-Von Arnim, Mclaughlin, Chapter 74 -Cellular Respiration
DOI record:
{
"DOI": "10.1007/s11095-021-03112-x",
"ISSN": [
"0724-8741",
"1573-904X"
],
"URL": "http://dx.doi.org/10.1007/s11095-021-03112-x",
"alternative-id": [
"3112"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "13 August 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "14 September 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "28 December 2021"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of Interest Statement",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Duke University has assigned the rights for the provisional patent applications to David Needham and his entity, Needham Material Science LLC."
},
{
"group": {
"label": "Author contributions for all authors",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "David Needham was the sole contributor and sole author."
},
{
"group": {
"label": "Data Availability Statement",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request."
},
{
"label": "Free to read",
"name": "free",
"value": "This content has been made available to all."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-0082-9148",
"affiliation": [],
"authenticated-orcid": false,
"family": "Needham",
"given": "David",
"sequence": "first"
}
],
"container-title": "Pharmaceutical Research",
"container-title-short": "Pharm Res",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2021,
12,
28
]
],
"date-time": "2021-12-28T16:02:27Z",
"timestamp": 1640707347000
},
"deposited": {
"date-parts": [
[
2022,
10,
28
]
],
"date-time": "2022-10-28T02:14:32Z",
"timestamp": 1666923272000
},
"indexed": {
"date-parts": [
[
2024,
3,
25
]
],
"date-time": "2024-03-25T13:14:37Z",
"timestamp": 1711372477301
},
"is-referenced-by-count": 11,
"issue": "1",
"issued": {
"date-parts": [
[
2021,
12,
28
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2022,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.springer.com/tdm",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
28
]
],
"date-time": "2021-12-28T00:00:00Z",
"timestamp": 1640649600000
}
},
{
"URL": "https://www.springer.com/tdm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
28
]
],
"date-time": "2021-12-28T00:00:00Z",
"timestamp": 1640649600000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s11095-021-03112-x.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s11095-021-03112-x/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s11095-021-03112-x.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"page": "115-141",
"prefix": "10.1007",
"published": {
"date-parts": [
[
2021,
12,
28
]
]
},
"published-online": {
"date-parts": [
[
2021,
12,
28
]
]
},
"published-print": {
"date-parts": [
[
2022,
1
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "3112_CR1",
"unstructured": "CDC. SARS-CoV-2 Variant Classifications and Definitions https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html 2021. Accessed on 2021–11–21."
},
{
"DOI": "10.1016/j.ijpharm.2003.09.035",
"author": "EC van Tonder",
"doi-asserted-by": "publisher",
"first-page": "417",
"issue": "2",
"journal-title": "Int J Pharm",
"key": "3112_CR2",
"unstructured": "van Tonder EC, Maleka TSP, Liebenberg W, Song M, Wurster DE, de Villiers MM. Preparation and physicochemical properties of niclosamide anhydrate and two monohydrates. Int J Pharm. 2004;269(2):417–32.",
"volume": "269",
"year": "2004"
},
{
"DOI": "10.1371/journal.pone.0246803",
"author": "AD Brunaugh",
"doi-asserted-by": "publisher",
"first-page": "e0246803-e",
"issue": "2",
"journal-title": "PloS One.",
"key": "3112_CR3",
"unstructured": "Brunaugh AD, Seo H, Warnken Z, Ding L, Seo SH, Smyth HDC. Development and evaluation of inhalable composite niclosamide-lysozyme particles: A broad-spectrum, patient-adaptable treatment for coronavirus infections and sequalae. PloS One. 2021;16(2):e0246803-e.",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03491-6",
"author": "L Braga",
"doi-asserted-by": "publisher",
"first-page": "88",
"issue": "7861",
"journal-title": "Nature",
"key": "3112_CR4",
"unstructured": "Braga L, Ali H, Secco I, Chiavacci E, Neves G, Goldhill D, et al. Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia. Nature. 2021;594(7861):88–93.",
"volume": "594",
"year": "2021"
},
{
"DOI": "10.1172/jci.insight.128414",
"doi-asserted-by": "crossref",
"key": "3112_CR5",
"unstructured": "Cabrita I, Benedetto R, Schreiber R, Kunzelmann K. Niclosamide repurposed for the treatment of inflammatory airway disease. JCI Insight. 2019;4(15)."
},
{
"DOI": "10.1371/journal.pone.0184324",
"author": "B Cheng",
"doi-asserted-by": "publisher",
"first-page": "e0184324-e",
"issue": "9",
"journal-title": "PloS One.",
"key": "3112_CR6",
"unstructured": "Cheng B, Morales LD, Zhang Y, Mito S, Tsin A. Niclosamide induces protein ubiquitination and inhibits multiple pro-survival signaling pathways in the human glioblastoma U-87 MG cell line. PloS One. 2017;12(9):e0184324-e.",
"volume": "12",
"year": "2017"
},
{
"DOI": "10.1128/AAC.01952-12",
"author": "F Imperi",
"doi-asserted-by": "publisher",
"first-page": "996",
"issue": "2",
"journal-title": "Antimicrob Agents Chemother",
"key": "3112_CR7",
"unstructured": "Imperi F, Massai F, Ramachandran Pillai C, Longo F, Zennaro E, Rampioni G, et al. New life for an old drug: the anthelmintic drug niclosamide inhibits Pseudomonas aeruginosa quorum sensing. Antimicrob Agents Chemother. 2013;57(2):996.",
"volume": "57",
"year": "2013"
},
{
"DOI": "10.1101/2020.03.20.999730",
"doi-asserted-by": "crossref",
"key": "3112_CR8",
"unstructured": "Jeon S, Ko M, Lee J, Choi I, Byun SY, Park S et al. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. bioRxiv. 2020:2020.03.20.999730."
},
{
"DOI": "10.1371/journal.ppat.1002976",
"author": "A Jurgeit",
"doi-asserted-by": "publisher",
"first-page": "e1002976",
"issue": "10",
"journal-title": "PLOS Pathogens.",
"key": "3112_CR9",
"unstructured": "Jurgeit A, McDowell R, Moese S, Meldrum E, Schwendener R, Greber UF. Niclosamide Is a Proton Carrier and Targets Acidic Endosomes with Broad Antiviral Effects. PLOS Pathogens. 2012;8(10):e1002976.",
"volume": "8",
"year": "2012"
},
{
"DOI": "10.1002/cmdc.201800100",
"author": "H Kadri",
"doi-asserted-by": "publisher",
"first-page": "1088",
"issue": "11",
"journal-title": "ChemMedChem",
"key": "3112_CR10",
"unstructured": "Kadri H, Lambourne OA, Mehellou Y. Niclosamide, a Drug with Many (Re)purposes. ChemMedChem. 2018;13(11):1088–91.",
"volume": "13",
"year": "2018"
},
{
"DOI": "10.1038/bcj.2011.38",
"author": "FL Khanim",
"doi-asserted-by": "publisher",
"first-page": "e39",
"journal-title": "Blood Cancer J",
"key": "3112_CR11",
"unstructured": "Khanim FL, Merrick BAME, Giles HV, Jankute M, Jackson JB, Giles LJ, et al. Redeployment-based drug screening identifies the anti-helminthic niclosamide as anti-myeloma therapy that also reduces free light chain production. Blood Cancer J. 2011;1:e39.",
"volume": "1",
"year": "2011"
},
{
"DOI": "10.1158/1078-0432.CCR-13-3296",
"author": "C Liu",
"doi-asserted-by": "publisher",
"first-page": "3198",
"issue": "12",
"journal-title": "Clin Cancer Res",
"key": "3112_CR12",
"unstructured": "Liu C, Lou W, Zhu Y, Nadiminty N, Schwartz CT, Evans CP, et al. Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer. Clin Cancer Res. 2014;20(12):3198–210.",
"volume": "20",
"year": "2014"
},
{
"DOI": "10.1016/j.reprotox.2016.11.005",
"author": "CW McCollum",
"doi-asserted-by": "publisher",
"first-page": "60",
"journal-title": "Reprod Toxicol",
"key": "3112_CR13",
"unstructured": "McCollum CW, Conde-Vancells J, Hans C, Vazquez-Chantada M, Kleinstreuer N, Tal T, et al. Identification of vascular disruptor compounds by analysis in zebrafish embryos and mouse embryonic endothelial cells. Reprod Toxicol. 2017;70:60–9.",
"volume": "70",
"year": "2017"
},
{
"DOI": "10.3389/fphar.2019.00051",
"doi-asserted-by": "crossref",
"key": "3112_CR14",
"unstructured": "Miner K, Labitzke K, Liu B, Wang P, Henckels K, Gaida K, et al. Drug Repurposing: The Anthelmintics Niclosamide and Nitazoxanide Are Potent TMEM16A Antagonists That Fully Bronchodilate Airways. Front Pharmacol. 2019;10(51)."
},
{
"DOI": "10.1158/1538-7445.AM2014-5491",
"author": "RA Mook",
"doi-asserted-by": "publisher",
"first-page": "5491",
"issue": "19 Supplement",
"journal-title": "Can Res",
"key": "3112_CR15",
"unstructured": "Mook RA, Zhao S, Chen W. Abstract 5491: Interrogating the mechanism of Wnt pathway inhibition by niclosamide. Can Res. 2014;74(19 Supplement):5491.",
"volume": "74",
"year": "2014"
},
{
"DOI": "10.3390/ph13120443",
"doi-asserted-by": "crossref",
"key": "3112_CR16",
"unstructured": "Mostafa A, Kandeil A, Elshaier AMMY, Kutkat O, Moatasim Y, Rashad AA, et al. FDA-Approved Drugs with Potent In Vitro Antiviral Activity against Severe Acute Respiratory Syndrome Coronavirus 2. Pharmaceuticals. 2020;13(12)."
},
{
"DOI": "10.1101/2020.12.16.422529",
"doi-asserted-by": "crossref",
"key": "3112_CR17",
"unstructured": "Prabhakara C, Godbole R, Sil P, Jahnavi S, van Zanten TS, Sheth D, et al. Niclosamide inhibits SARS-CoV2 entry by blocking internalization through pH-dependent CLIC/GEEC endocytic pathway. bioRxiv. 2020:2020.12.16.422529."
},
{
"DOI": "10.1371/journal.pone.0198389",
"author": "MT Schweizer",
"doi-asserted-by": "publisher",
"first-page": "e0198389",
"issue": "6",
"journal-title": "PLOS ONE.",
"key": "3112_CR18",
"unstructured": "Schweizer MT, Haugk K, McKiernan JS, Gulati R, Cheng HH, Maes JL, et al. A phase I study of niclosamide in combination with enzalutamide in men with castration-resistant prostate cancer. PLOS ONE. 2018;13(6):e0198389.",
"volume": "13",
"year": "2018"
},
{
"DOI": "10.1021/acsinfecdis.0c00052",
"author": "J Xu",
"doi-asserted-by": "publisher",
"first-page": "909",
"issue": "5",
"journal-title": "ACS Infect Dis",
"key": "3112_CR19",
"unstructured": "Xu J, Shi P-Y, Li H, Zhou J. Broad Spectrum Antiviral Agent Niclosamide and Its Therapeutic Potential. ACS Infect Dis. 2020;6(5):909–15.",
"volume": "6",
"year": "2020"
},
{
"key": "3112_CR20",
"unstructured": "Bayer. YOMESAN® tablets (MSDS) https://www.bayer.co.za/static/documents/MSDS/PIs/YOMESAN_EN_PI.pdf. 2008 Accessed on 2021–11–21."
},
{
"author": "GJ Abrams",
"first-page": "6",
"journal-title": "S Afr Med J",
"key": "3112_CR21",
"unstructured": "Abrams GJ, Seftel HC, Heinz HJ. The treatment of human tapeworm infections with ‘Yomesan.’ S Afr Med J. 1963;37:6–8.",
"volume": "37",
"year": "1963"
},
{
"DOI": "10.1016/j.cellsig.2017.04.001",
"author": "W Chen",
"doi-asserted-by": "publisher",
"first-page": "89",
"journal-title": "Cell Signal",
"key": "3112_CR22",
"unstructured": "Chen W, Mook RA, Premont RT, Wang J. Niclosamide: Beyond an antihelminthic drug. Cell Signal. 2018;41:89–96.",
"volume": "41",
"year": "2018"
},
{
"key": "3112_CR23",
"unstructured": "Goulding J. Virus Replication https://www.immunology.org/public-information/bitesized-immunology/pathogens-and-disease/virus-replication. British Society for Immunology 2021. Accessd on 2021–11–21."
},
{
"DOI": "10.1101/2020.05.12.091256",
"doi-asserted-by": "crossref",
"key": "3112_CR24",
"unstructured": "Laise P, Bosker G, Sun X, Shen Y, Douglass EF, Karan C, et al. The Host Cell ViroCheckpoint: Identification and Pharmacologic Targeting of Novel Mechanistic Determinants of Coronavirus-Mediated Hijacked Cell States. bioRxiv : the preprint server for biology. 2020:2020.05.12.091256."
},
{
"key": "3112_CR25",
"unstructured": "Needham D. A White Paper. Why Niclosamide? A Basis for New Nasal and Throat Spray Solution-Formulation for COVID19 and Other Viral Infections. In preparation, to be submitted to Pharmaceutical Research. 2021."
},
{
"DOI": "10.1164/rccm.202003-0572LE",
"author": "Y Huang",
"doi-asserted-by": "publisher",
"first-page": "1435",
"issue": "11",
"journal-title": "Am J Respir Crit Care Med",
"key": "3112_CR26",
"unstructured": "Huang Y, Chen S, Yang Z, Guan W, Liu D, Lin Z, et al. SARS-CoV-2 Viral Load in Clinical Samples from Critically Ill Patients. Am J Respir Crit Care Med. 2020;201(11):1435–8.",
"volume": "201",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2003138117",
"author": "J Shang",
"doi-asserted-by": "publisher",
"first-page": "11727",
"issue": "21",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "3112_CR27",
"unstructured": "Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117(21):11727–34.",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1038/s41598-019-45095-1",
"author": "E Jung",
"doi-asserted-by": "publisher",
"first-page": "8682",
"issue": "1",
"journal-title": "Sci Rep",
"key": "3112_CR28",
"unstructured": "Jung E, Nam S, Oh H, Jun S, Ro H-J, Kim B, et al. Neutralization of Acidic Intracellular Vesicles by Niclosamide Inhibits Multiple Steps of the Dengue Virus Life Cycle In Vitro. Sci Rep. 2019;9(1):8682.",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1016/S0021-9258(18)69162-8",
"author": "RC Conaway",
"doi-asserted-by": "publisher",
"first-page": "2962",
"issue": "6",
"journal-title": "J Biol Chem",
"key": "3112_CR29",
"unstructured": "Conaway RC, Conaway JW. ATP activates transcription initiation from promoters by RNA polymerase II in a reversible step prior to RNA synthesis. J Biol Chem. 1988;263(6):2962–8.",
"volume": "263",
"year": "1988"
},
{
"DOI": "10.1016/B978-0-323-07307-3.10074-6",
"doi-asserted-by": "crossref",
"key": "3112_CR30",
"unstructured": "Zimmerman JJ, von Saint André-von Arnim A, McLaughlin J. Chapter 74 - Cellular Respiration. In: Fuhrman BP, Zimmerman JJ, editors. Pediatric Critical Care (Fourth Edition). Saint Louis: Mosby; 2011. p. 1058–72."
},
{
"key": "3112_CR31",
"unstructured": "Promega. CellTiter-Glo® 2.0 Cell Viability Assay. https://www.promega.com/products/cell-health-assays/cell-viability-and-cytotoxicity-assays/celltiter_glo-2_0-assay. 2021 (Accessd on 2021–11–21)."
},
{
"DOI": "10.1186/1423-0127-16-80",
"author": "C-W Chang",
"doi-asserted-by": "publisher",
"first-page": "80-",
"issue": "1",
"journal-title": "J Biomed Sci.",
"key": "3112_CR32",
"unstructured": "Chang C-W, Li H-C, Hsu C-F, Chang C-Y, Lo S-Y. Increased ATP generation in the host cell is required for efficient vaccinia virus production. J Biomed Sci. 2009;16(1):80-.",
"volume": "16",
"year": "2009"
},
{
"DOI": "10.3390/v12010056",
"author": "PD Nagy",
"doi-asserted-by": "publisher",
"first-page": "56",
"journal-title": "Viruses",
"key": "3112_CR33",
"unstructured": "Nagy PD, Lin W. Taking over Cellular Energy-Metabolism for TBSV Replication: The High ATP Requirement of an RNA Virus within the Viral Replication Organelle. Viruses. 2020;12:56.",
"volume": "12",
"year": "2020"
},
{
"key": "3112_CR34",
"unstructured": "Kim S, Bear S, Needham D, Lee P. Effect of Niclosamide on cytotoxicity and cell viability in small airway epithelial cells in preparation. 2021."
},
{
"DOI": "10.1101/2020.05.12.090035",
"doi-asserted-by": "crossref",
"key": "3112_CR35",
"unstructured": "Ko M, Jeon S, Ryu W-S, Kim S. Comparative analysis of antiviral efficacy of FDA-approved drugs against SARS-CoV-2 in human lung cells: Nafamostat is the most potent antiviral drug candidate. bioRxiv. 2020:2020.05.12.090035."
},
{
"key": "3112_CR36",
"unstructured": "clinicaltrials.gov. COVID19 Niclosamide https://clinicaltrials.gov/ct2/results?term=Niclosamide&cond=Covid19. 2021 Accessd on 2021-11-21."
},
{
"key": "3112_CR37",
"unstructured": "Daewoong-Pharmaceutical. Daewoong Pharmaceutical Partners with Tufts Medical Center for Phase 2 Clinical Trial with Niclosamide. https://www.prnewswire.com/news-releases/daewoong-pharmaceutical-partners-with-tufts-medical-center-for-phase-2-clinical-trial-with-niclosamide-301162739.html. 2020 Accessed on 2021–11–21."
},
{
"key": "3112_CR38",
"unstructured": "Union-Therapeutics. UNION’s nasal spray for COVID-19 enters a 1,500 high-risk patient study. 2021."
},
{
"DOI": "10.1111/bcpt.12342",
"author": "M Boegh",
"doi-asserted-by": "publisher",
"first-page": "179",
"issue": "3",
"journal-title": "Basic Clin Pharmacol Toxicol",
"key": "3112_CR39",
"unstructured": "Boegh M, Nielsen HM. Mucus as a barrier to drug delivery – understanding and mimicking the barrier properties. Basic Clin Pharmacol Toxicol. 2015;116(3):179–86.",
"volume": "116",
"year": "2015"
},
{
"DOI": "10.1016/j.ijpharm.2017.09.018",
"author": "J Leal",
"doi-asserted-by": "publisher",
"first-page": "555",
"issue": "1",
"journal-title": "Int J Pharm",
"key": "3112_CR40",
"unstructured": "Leal J, Smyth HDC, Ghosh D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int J Pharm. 2017;532(1):555–72.",
"volume": "532",
"year": "2017"
},
{
"DOI": "10.25004/IJPSDR.2017.090603",
"author": "AD Paul",
"doi-asserted-by": "publisher",
"first-page": "299",
"journal-title": "Int J Pharm Sci Drug Res",
"key": "3112_CR41",
"unstructured": "Paul AD, Samatha P, Manasa SL, Munemma R, Supriya D. Modeling the oral cavity with mucoadhesive drug delivery systems - a potential alternative to conventional therapy. Int J Pharm Sci Drug Res. 2017;9:299–307.",
"volume": "9",
"year": "2017"
},
{
"DOI": "10.1016/j.cell.2020.05.042",
"author": "YJ Hou",
"doi-asserted-by": "publisher",
"first-page": "429",
"issue": "2",
"journal-title": "Cell",
"key": "3112_CR42",
"unstructured": "Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH 3rd, et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell. 2020;182(2):429-46.e14.",
"volume": "182",
"year": "2020"
},
{
"DOI": "10.1016/S0378-5173(99)00442-1",
"author": "N Washington",
"doi-asserted-by": "publisher",
"first-page": "139",
"issue": "2",
"journal-title": "Int J Pharm",
"key": "3112_CR43",
"unstructured": "Washington N, Steele RJ, Jackson SJ, Bush D, Mason J, Gill DA, et al. Determination of baseline human nasal pH and the effect of intranasally administered buffers. Int J Pharm. 2000;198(2):139–46.",
"volume": "198",
"year": "2000"
},
{
"key": "3112_CR44",
"unstructured": "Shah BAK. FLONASE (fluticasone propionate) nasal spray. Prescribing information. In: DPARP, editor. 2019."
},
{
"DOI": "10.2165/00003495-200868120-00009",
"author": "CM Baldwin",
"doi-asserted-by": "publisher",
"first-page": "1723",
"issue": "12",
"journal-title": "Drugs",
"key": "3112_CR45",
"unstructured": "Baldwin CM, Scott LJ. Mometasone furoate: a review of its intranasal use in allergic rhinitis. Drugs. 2008;68(12):1723–39.",
"volume": "68",
"year": "2008"
},
{
"key": "3112_CR46",
"unstructured": "Merck. Prescribing information Nasonex. 1997."
},
{
"DOI": "10.1016/S0169-409X(01)00172-7",
"author": "N Jones",
"doi-asserted-by": "publisher",
"first-page": "5",
"issue": "1",
"journal-title": "Adv Drug Deliv Rev",
"key": "3112_CR47",
"unstructured": "Jones N. The nose and paranasal sinuses physiology and anatomy. Adv Drug Deliv Rev. 2001;51(1):5–19.",
"volume": "51",
"year": "2001"
},
{
"key": "3112_CR48",
"unstructured": "Needham D, inventor; Duke University, assignee. Current Provisional Patent Portfolio USPO: a) DU7305PROV, US 63/111,184 “ANTI-VIRAL NICLOSAMIDE COMPOSITIONS AS VIROSTATIC THERAPEUTICS AND METHODS OF MAKING AND USING SAME” (filed 11/9/2020). b) DU7185PROV, US 63/049,160, “NICLOSASPRAY” AND “NICLOSADROPS” FOR BUCCAL/THROAT, NASAL, AND EYE DROPS AND METHODS OF USING SAME” (filed 7/8/2020). c) DU7175PROV, US 63/049,153, “ANTIVIRAL NICLOSAMIDE COMPOSITIONS AS VIROSTATIC THERAPEUTICS AND METHODS OF MAKING AND USING SAME” (filed 7/8/2020). d) DU7109PROV, US 63/021,250, “COMPOSITIONS COMPRISING NICLOSAMIDE AND METHODS OF USING SAME AS PROPHYLAXIS AND TREATMENT OF VIRAL INFECTIONS” (filed 5/7/2020). e) DU6187PROV, US 63/021,241, PRECIPITATION OF HYDROPHOBIC DRUGS AS NANOCRYSTALS FOR INJECTION TO A SUBJECT AND METHODS OF USING SAME (filed 5/72020). f) DU7099PROV, US 63/014,748, “COMPOSITIONS COMPRISING NICLOSAMIDE AND METHODS OF MAKING AND USING SAME FOR THE PROPHYLAXIS AND TREATMENT OF VIRAL INFECTIONS” (filed 4/24/2020). g) DU7065PROV2, US 63/111,785, “COMPOSITIONS AND FORMULATIONS COMPRISING NICLOSAMIDE AND METHODS OF MAKING AND USING SAME FOR THE TREATMENT OF VIRAL INFECTIONS” (filed 4/17/2020). h) DU7065PROV, US 63/006,527, “NICLOSAMIDE COMPOSITIONS AND METHODS OF USING SAME FOR THE TREATMENT OF VIRAL INFECTIONS” (filed 4/7/2020). i) DU7308PROV, US 63/007,007, “ANTI-VIRAL NICLOSAMIDE COMPOSITIONS AS VIROSTATIC THERAPEUTICS AND METHODS OF MAKING AND USING SAME” (filed 3/26/2020). USA2020."
},
{
"DOI": "10.1128/AAC.00819-20",
"author": "S Jeon",
"doi-asserted-by": "publisher",
"first-page": "e00819",
"issue": "7",
"journal-title": "Antimicrob Agents Chemother",
"key": "3112_CR49",
"unstructured": "Jeon S, Ko M, Lee J, Choi I, Byun SY, Park S, et al. Identification of Antiviral Drug Candidates against SARS-CoV-2 from FDA-Approved Drugs. Antimicrob Agents Chemother. 2020;64(7):e00819-e820.",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.15585/mmwr.mm7021e3",
"doi-asserted-by": "crossref",
"key": "3112_CR50",
"unstructured": "CDC. COVID-19 Vaccine Breakthrough Infections Reported to CDC — United States, January 1–April 30, 2021, https://www.cdc.gov/mmwr/volumes/70/wr/mm7021e3.htm. 2021 Accessed on 2021–11–21."
},
{
"key": "3112_CR51",
"unstructured": "Delta Variant Key to Breakthrough Infections in Vaccinated Israelis [press release]. Medscape2021."
},
{
"key": "3112_CR52",
"unstructured": "Ourworldindata. Share of people vaccinated against COVID-19, Jul 23, 2021. 2021."
},
{
"key": "3112_CR53",
"unstructured": "Hernandez J. Nearly 94,000 Kids Got COVID-19 Last Week. They Were 15% Of All New Cases (August 10 2021). NPR; 2021."
},
{
"DOI": "10.1080/1061186X.2016.1238092",
"author": "D Needham",
"doi-asserted-by": "publisher",
"first-page": "836",
"issue": "9",
"journal-title": "J Drug Target",
"key": "3112_CR54",
"unstructured": "Needham D, Arslanagic A, Glud K, Hervella P, Karimi L, Høeilund-Carlsen P-F, et al. Bottom up design of nanoparticles for anti-cancer diapeutics. J Drug Target. 2016;24(9):836–56.",
"volume": "24",
"year": "2016"
},
{
"DOI": "10.1158/1535-7163.MCT-19-0689",
"author": "GB Reddy",
"doi-asserted-by": "publisher",
"first-page": "1448",
"issue": "7",
"journal-title": "Mol Cancer Ther.",
"key": "3112_CR55",
"unstructured": "Reddy GB, Kerr DL, Spasojevic I, Tovmasyan A, Hsu D, Brigman BE, et al. Preclinical Testing of a Novel Niclosamide Stearate Prodrug Therapeutic (NSPT) shows efficacy against Osteosarcoma. Mol Cancer Ther. 2020;19(7):1448.",
"volume": "19",
"year": "2020"
},
{
"key": "3112_CR56",
"unstructured": "FAO. Food and Agriculture Organization of the United Nations (FAO) SPECIFICATIONS AND EVALUATIONS FOR AGRICULTURAL PESTICIDES NICLOSAMIDE 2’,5-dichloro-4’-nitrosalicylanilide 2004."
},
{
"DOI": "10.1021/acs.est.6b02607",
"author": "MB McConville",
"doi-asserted-by": "publisher",
"first-page": "9998",
"issue": "18",
"journal-title": "Environ Sci Technol",
"key": "3112_CR57",
"unstructured": "McConville MB, Hubert TD, Remucal CK. Direct Photolysis Rates and Transformation Pathways of the Lampricides TFM and Niclosamide in Simulated Sunlight. Environ Sci Technol. 2016;50(18):9998–10006.",
"volume": "50",
"year": "2016"
},
{
"DOI": "10.1016/j.chemosphere.2018.03.021",
"author": "TH Tran",
"doi-asserted-by": "publisher",
"first-page": "425",
"journal-title": "Chemosphere",
"key": "3112_CR58",
"unstructured": "Tran TH, Labanowski J, Gallard H. Adsorption and transformation of the anthelmintic drug niclosamide by manganese oxide. Chemosphere. 2018;201:425–31.",
"volume": "201",
"year": "2018"
},
{
"DOI": "10.1023/A:1007931314609",
"author": "MR Caira",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "1",
"journal-title": "J Inclusion Phenom Mol Recognit Chem",
"key": "3112_CR59",
"unstructured": "Caira MR, Van Tonder EC, De Villiers MM, Lötter AP. Diverse Modes of Solvent Inclusion in Crystalline Pseudopolymorphs of the Anthelmintic Drug Niclosamide. J Inclusion Phenom Mol Recognit Chem. 1998;31(1):1–16.",
"volume": "31",
"year": "1998"
},
{
"DOI": "10.1081/DDC-120037489",
"author": "MM de Villiers",
"doi-asserted-by": "publisher",
"first-page": "581",
"issue": "6",
"journal-title": "Drug Dev Ind Pharm",
"key": "3112_CR60",
"unstructured": "de Villiers MM, Mahlatji MD, van Tonder EC, Malan SF, Lötter AP, Liebenberg W. Comparison of the physical and chemical stability of niclosamide crystal forms in aqueous versus nonaqueous suspensions. Drug Dev Ind Pharm. 2004;30(6):581–92.",
"volume": "30",
"year": "2004"
},
{
"DOI": "10.1007/BF02830580",
"author": "EC van Tonder",
"doi-asserted-by": "publisher",
"first-page": "E12-E",
"issue": "1",
"journal-title": "AAPS PharmSciTech.",
"key": "3112_CR61",
"unstructured": "van Tonder EC, Mahlatji MD, Malan SF, Liebenberg W, Caira MR, Song M, et al. Preparation and physicochemical characterization of 5 niclosamide solvates and 1 hemisolvate. AAPS PharmSciTech. 2004;5(1):E12-E.",
"volume": "5",
"year": "2004"
},
{
"key": "3112_CR62",
"unstructured": "Burley J, Needham D. Characterization of Niclosamide (XRD, FT-IR/Raman, DSC) from various suppliers and as precipitates and cosolvates confirms polymorphic forms as a function of pH (in preparation). 2021."
},
{
"DOI": "10.1119/1.2772287",
"author": "G Halász",
"doi-asserted-by": "publisher",
"first-page": "1092",
"journal-title": "Am J Phys",
"key": "3112_CR63",
"unstructured": "Halász G, Gyüre B, Jánosi IM. Vortex flow generated by a magnetic stirrer. Am J Phys. 2007;75:1092.",
"volume": "75",
"year": "2007"
},
{
"DOI": "10.1021/la034930i",
"author": "PB Duncan",
"doi-asserted-by": "publisher",
"first-page": "2567",
"issue": "7",
"journal-title": "Langmuir",
"key": "3112_CR64",
"unstructured": "Duncan PB, Needham D. Test of the Epstein-Plesset model for gas microparticle dissolution in aqueous media: Effect of surface tension and gas undersaturation in solution. Langmuir. 2004;20(7):2567–78.",
"volume": "20",
"year": "2004"
},
{
"DOI": "10.1021/la053314e",
"author": "PB Duncan",
"doi-asserted-by": "publisher",
"first-page": "4190",
"issue": "9",
"journal-title": "Langmuir",
"key": "3112_CR65",
"unstructured": "Duncan PB, Needham D. Microdroplet dissolution into a second-phase solvent using a micropipet technique: Test of the Epstein-Plesset model for an aniline-water system. Langmuir. 2006;22(9):4190–7.",
"volume": "22",
"year": "2006"
},
{
"DOI": "10.1063/1.1747520",
"author": "PS Epstein",
"doi-asserted-by": "publisher",
"first-page": "1505",
"issue": "11",
"journal-title": "J Chem Phys",
"key": "3112_CR66",
"unstructured": "Epstein PS, Plesset MS. On the Stability of Gas Bubbles in Liquid-Gas Solutions. J Chem Phys. 1950;18(11):1505–2409.",
"volume": "18",
"year": "1950"
},
{
"DOI": "10.1021/la402533j",
"author": "JT Su",
"doi-asserted-by": "publisher",
"first-page": "13339",
"issue": "44",
"journal-title": "Langmuir",
"key": "3112_CR67",
"unstructured": "Su JT, Needham D. Mass transfer in the dissolution of a multicomponent liquid droplet in an immiscible liquid environment. Langmuir. 2013;29(44):13339–45.",
"volume": "29",
"year": "2013"
},
{
"DOI": "10.1021/la034779c",
"author": "DH Kim",
"doi-asserted-by": "publisher",
"first-page": "8455",
"issue": "20",
"journal-title": "Langmuir",
"key": "3112_CR68",
"unstructured": "Kim DH, Costello MJ, Duncan PB, Needham D. Mechanical properties and microstructure of polycrystalline phospholipid monolayer shells: Novel solid microparticles. Langmuir. 2003;19(20):8455–66.",
"volume": "19",
"year": "2003"
},
{
"author": "DH Kim",
"first-page": "A313-A",
"issue": "2",
"journal-title": "Biophys J.",
"key": "3112_CR69",
"unstructured": "Kim DH, Klibanov AL, Evans EA, Needham D. Viscoelastic properties of phospholipid monolayer shells on air microbubbles. Biophys J. 1998;74(2):A313-A.",
"volume": "74",
"year": "1998"
},
{
"DOI": "10.1107/S0021889807007832",
"author": "A Llinàs",
"doi-asserted-by": "publisher",
"first-page": "379",
"issue": "2",
"journal-title": "J Appl Crystallogr",
"key": "3112_CR70",
"unstructured": "Llinàs A, Box KJ, Burley JC, Glen RC, Goodman JM. A new method for the reproducible generation of polymorphs: two forms of sulindac with very different solubilities. J Appl Crystallogr. 2007;40(2):379–81.",
"volume": "40",
"year": "2007"
},
{
"DOI": "10.1101/2021.01.26.428293",
"doi-asserted-by": "crossref",
"key": "3112_CR71",
"unstructured": "Jara MO, Warnken ZN, Sahakijpijarn S, Moon C, Maier EY, Christensen DJ, et al. Niclosamide Inhalation Powder Made by Thin-Film Freezing: Pharmacokinetic and Toxicology Studies in Rats and Hamsters. bioRxiv. 2021:2021.01.26.428293."
},
{
"key": "3112_CR72",
"unstructured": "Union-Therapeutics. UNION therapeutics A/S completes dosing of healthy volunteers with UNI911 (inhaled niclosamide) for COVID-19 https://www.uniontherapeutics.com/news-events/news/union-therapeutics-a-s-completes-dosing-of-healthy-volunteers-with-uni911-inhaled-niclosamide-for-covid-19. 2020 Accessed on 2021–11–21."
},
{
"key": "3112_CR73",
"unstructured": "CDC. Global COVID-19 Vaccinations https://covid.cdc.gov/covid-data-tracker/#global-vaccinations. 2021 Accessed on 2021–11–21."
}
],
"reference-count": 73,
"references-count": 73,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2021.08.16.456531",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s11095-021-03112-x"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Organic Chemistry",
"Pharmaceutical Science",
"Pharmacology",
"Molecular Medicine",
"Biotechnology"
],
"subtitle": [],
"title": "The pH Dependence of Niclosamide Solubility, Dissolution, and Morphology: Motivation for Potentially Universal Mucin-Penetrating Nasal and Throat Sprays for COVID19, its Variants and other Viral Infections",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "39"
}