Investigating efficacy of colchicine plus phenolic monoterpenes fraction as a potential treatment for patients diagnosed with COVID-19: A randomized controlled parallel clinical trial
et al., Heliyon, doi:10.1016/j.heliyon.2024.e27373, NCT04392141, Mar 2024
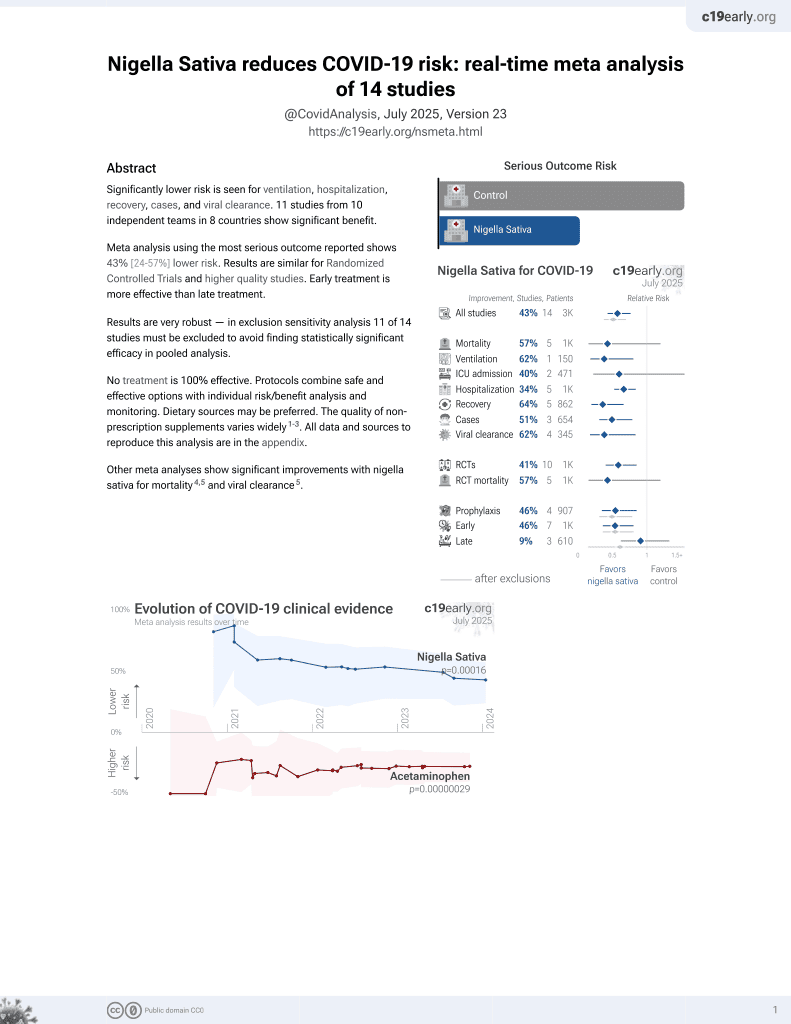
14th treatment shown to reduce risk in
January 2021, now with p = 0.00016 from 14 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 179 hospitalized COVID-19 patients showing lower mortality, ICU admission, and hospitalization duration with colchicine plus phenolic monoterpenes compared to standard care alone. The intervention group received 0.8 mg/day colchicine and 45 mg/day phenolic monoterpenes extracted from nigella sativa and Trachyspermum ammi in addition to standard care (lopinavir/ritonavir). No serious side effects were reported. Baseline SpO2 was significantly lower in the control group, although there was no significant difference in severity according to NIH guidelines.
Study covers nigella sativa and colchicine.
|
risk of death, 81.2% lower, RR 0.19, p = 0.03, treatment 2 of 108 (1.9%), control 7 of 71 (9.9%), NNT 12, after 14 day followup.
|
|
risk of death, 89.0% lower, RR 0.11, p = 0.02, treatment 1 of 108 (0.9%), control 6 of 71 (8.5%), NNT 13, in hospital.
|
|
risk of ICU admission, 86.9% lower, RR 0.13, p = 0.002, treatment 2 of 108 (1.9%), control 10 of 71 (14.1%), NNT 8.2.
|
|
hospitalization time, 34.7% lower, relative time 0.65, p < 0.001, treatment mean 4.17 (±1.34) n=108, control mean 6.39 (±2.59) n=71.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Vaziri et al., 6 Mar 2024, Randomized Controlled Trial, Iran, peer-reviewed, mean age 54.2, 11 authors, study period April 2020 - December 2020, this trial uses multiple treatments in the treatment arm (combined with colchicine and phenolic monoterpenes from nigella sativa and Trachyspermum ammi) - results of individual treatments may vary, trial NCT04392141 (history).
Contact: amostafaie@kums.ac.ir.
DOI record:
{
"DOI": "10.1016/j.heliyon.2024.e27373",
"ISSN": [
"2405-8440"
],
"URL": "http://dx.doi.org/10.1016/j.heliyon.2024.e27373",
"alternative-id": [
"S2405844024034042"
],
"article-number": "e27373",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Investigating efficacy of colchicine plus phenolic monoterpenes fraction as a potential treatment for patients diagnosed with COVID-19: A randomized controlled parallel clinical trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Heliyon"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.heliyon.2024.e27373"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 The Authors. Published by Elsevier Ltd."
}
],
"author": [
{
"affiliation": [],
"family": "Vaziri",
"given": "Siavash",
"sequence": "first"
},
{
"affiliation": [],
"family": "Janbakhsh",
"given": "Alireza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zamanian",
"given": "Mohammad Hossein",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shakiba",
"given": "Yadollah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mostafaei",
"given": "Shayan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Norooznezhad",
"given": "Amir Hossein",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mansouri",
"given": "Kamran",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bagheri",
"given": "Ahmad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abdali",
"given": "Farhad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fatahpour",
"given": "Kavyan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mostafaie",
"given": "Ali",
"sequence": "additional"
}
],
"container-title": "Heliyon",
"container-title-short": "Heliyon",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"cell.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
3,
6
]
],
"date-time": "2024-03-06T08:28:28Z",
"timestamp": 1709713708000
},
"deposited": {
"date-parts": [
[
2024,
3,
6
]
],
"date-time": "2024-03-06T08:28:35Z",
"timestamp": 1709713715000
},
"indexed": {
"date-parts": [
[
2024,
3,
7
]
],
"date-time": "2024-03-07T00:33:10Z",
"timestamp": 1709771590804
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
3
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
3,
1
]
],
"date-time": "2024-03-01T00:00:00Z",
"timestamp": 1709251200000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
28
]
],
"date-time": "2024-02-28T00:00:00Z",
"timestamp": 1709078400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2405844024034042?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2405844024034042?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "e27373",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
3
]
]
},
"published-print": {
"date-parts": [
[
2024,
3
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.4269/ajtmh.20-0512",
"article-title": "Primary symptoms, comorbidities, and outcomes of 431 hospitalized patients with confirmative RT-PCR results for COVID-19",
"author": "Norooznezhad",
"doi-asserted-by": "crossref",
"first-page": "834",
"journal-title": "Am. J. Trop. Med. Hyg.",
"key": "10.1016/j.heliyon.2024.e27373_bib1",
"volume": "103",
"year": "2020"
},
{
"DOI": "10.1016/j.arcmed.2020.03.015",
"article-title": "Inappropriate antibiotic consumption as a possible cause of inflammatory storm and septic shock in patients diagnosed with coronavirus-19 disease (COVID-19)",
"author": "Hantoushzadeh",
"doi-asserted-by": "crossref",
"first-page": "347",
"journal-title": "Arch. Med. Res.",
"key": "10.1016/j.heliyon.2024.e27373_bib2",
"volume": "51",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "Lancet",
"key": "10.1016/j.heliyon.2024.e27373_bib3",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1002/jmv.26525",
"article-title": "Changes of leukocytes, neutrophils, and lymphocytes count and dependent variables in pregnant women with coronavirus disease 2019 (COVID-19) before and after cesarean delivery",
"author": "Norooznezhad",
"doi-asserted-by": "crossref",
"first-page": "664",
"journal-title": "J. Med. Virol.",
"key": "10.1016/j.heliyon.2024.e27373_bib4",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.3390/jcm9051583",
"article-title": "Herbal medicine for the treatment of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis of randomized controlled trials",
"author": "Ang",
"doi-asserted-by": "crossref",
"first-page": "1583",
"journal-title": "J. Clin. Med.",
"key": "10.1016/j.heliyon.2024.e27373_bib5",
"volume": "9",
"year": "2020"
},
{
"key": "10.1016/j.heliyon.2024.e27373_bib6",
"series-title": "Definitions of Symptoms for Reportable Illnesses",
"year": "2020"
},
{
"key": "10.1016/j.heliyon.2024.e27373_bib8",
"series-title": "Management of Persons with COVID-19",
"year": "2020"
},
{
"article-title": "Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial",
"author": "Deftereos",
"journal-title": "JAMA New Open",
"key": "10.1016/j.heliyon.2024.e27373_bib9",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1136/rmdopen-2020-001455",
"article-title": "Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial",
"author": "Lopes",
"doi-asserted-by": "crossref",
"journal-title": "RMD Open",
"key": "10.1016/j.heliyon.2024.e27373_bib10",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1186/s13063-020-04647-x",
"article-title": "Nigella sativa supplementation to treat symptomatic mild COVID-19: a structured summary of a protocol for a randomised, controlled, clinical trial",
"author": "Koshak",
"doi-asserted-by": "crossref",
"first-page": "703",
"journal-title": "Trials",
"key": "10.1016/j.heliyon.2024.e27373_bib11",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1016/j.semarthrit.2015.06.013",
"article-title": "Colchicine--Update on mechanisms of action and therapeutic uses",
"author": "Leung",
"doi-asserted-by": "crossref",
"first-page": "341",
"journal-title": "Semin. Arthritis Rheum.",
"key": "10.1016/j.heliyon.2024.e27373_bib12",
"volume": "45",
"year": "2015"
},
{
"DOI": "10.2174/1871529X18666180212114816",
"article-title": "Immunomodulatory and anti-inflammatory effects of thymoquinone",
"author": "Shaterzadeh-Yazdi",
"doi-asserted-by": "crossref",
"first-page": "52",
"journal-title": "Cardiovasc. Hematol. Disord.: Drug Targets",
"key": "10.1016/j.heliyon.2024.e27373_bib13",
"volume": "18",
"year": "2018"
},
{
"DOI": "10.3390/antiox9090897",
"article-title": "Implications of oxidative stress and potential role of mitochondrial dysfunction in COVID-19: therapeutic effects of vitamin D",
"author": "de Las Heras",
"doi-asserted-by": "crossref",
"first-page": "897",
"journal-title": "Antioxidants",
"key": "10.1016/j.heliyon.2024.e27373_bib14",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1093/eurheartj/ehaa623",
"article-title": "COVID-19 is, in the end, an endothelial disease",
"author": "Libby",
"doi-asserted-by": "crossref",
"first-page": "3038",
"journal-title": "Eur. Heart J.",
"key": "10.1016/j.heliyon.2024.e27373_bib15",
"volume": "41",
"year": "2020"
}
],
"reference-count": 14,
"references-count": 14,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2405844024034042"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "Investigating efficacy of colchicine plus phenolic monoterpenes fraction as a potential treatment for patients diagnosed with COVID-19: A randomized controlled parallel clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}