Treating Covid-19 With Hydroxychloroquine (TEACH): A Multicenter, Double-Blind, Randomized Controlled Trial in Hospitalized Patients
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofaa446, TEACH, NCT04369742, Sep 2020
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
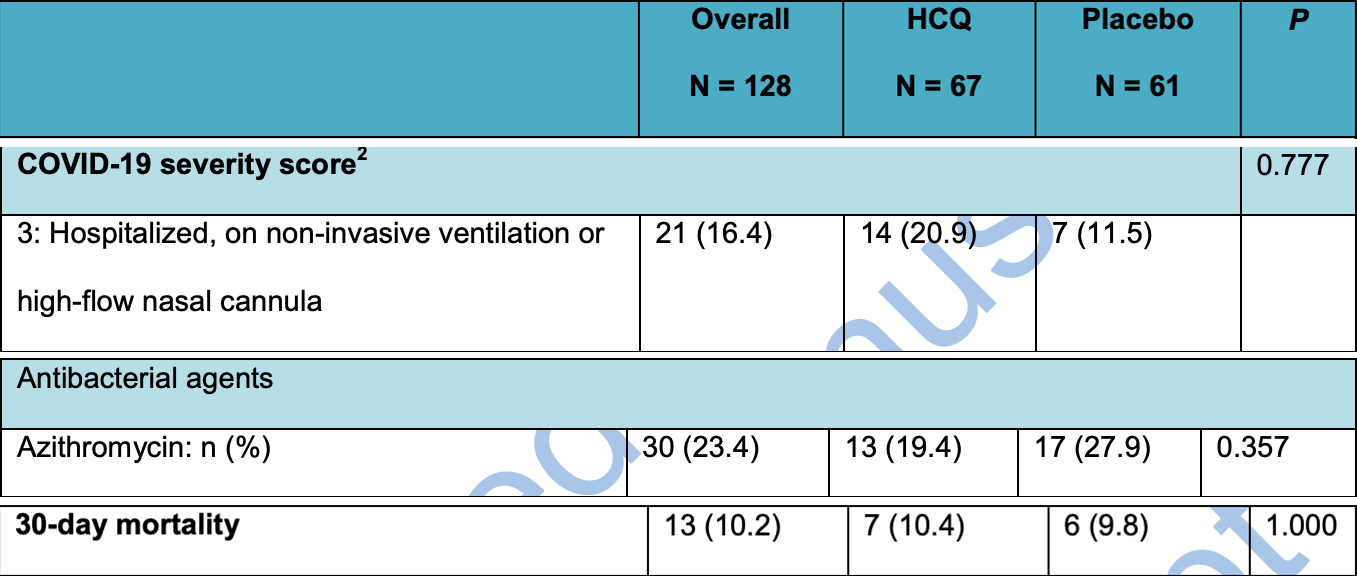
Small RCT on very late stage use of HCQ, with 48% on oxygen at baseline. 67 HCQ patients, 61 control. Baseline states were not comparable - 82% more HCQ patients had the highest severity at baseline, there was 32% more male HCQ patients, and 44% more control patients used AZ. The HCQ group also had significantly more patients with cerebrovascular disease, cardiovascular disease (non-hypertension), renal disease (non-dialysis), and a history of organ transplants.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This study is excluded in the after exclusion results of meta-analysis:
very late stage, >50% on oxygen/ventilation at baseline.
|
risk of death, 6.0% higher, RR 1.06, p = 1.00, treatment 7 of 67 (10.4%), control 6 of 61 (9.8%).
|
|
risk of mechanical ventilation, 51.7% higher, RR 1.52, p = 0.72, treatment 5 of 67 (7.5%), control 3 of 61 (4.9%).
|
|
risk of ICU admission, 173.1% higher, RR 2.73, p = 0.13, treatment 9 of 67 (13.4%), control 3 of 61 (4.9%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Ulrich et al., 23 Sep 2020, Randomized Controlled Trial, USA, peer-reviewed, baseline oxygen required 63.3%, mean age 66.2, 18 authors, study period 17 April, 2020 - 12 May, 2020, average treatment delay 7.0 days, trial NCT04369742 (history) (TEACH).
TREATING COVID-19 WITH HYDROXYCHLOROQUINE (TEACH): A MULTICENTER, DOUBLE-BLIND, RANDOMIZED CONTROLLED TRIAL IN HOSPITALIZED PATIENTS
doi:10.1093/ofid/ofaa446/5910201
Author Contributions: RJU, ABT, EC, VR and MJM contributed to the concept, design and protocol development. RJU, EC, JE, MB, JAD and PJP contributed as site leaders overseeing all trial operations and data quality from each site. MJ, GAR, BH, AH, DD and YD contributed to trial operations and data entry. RJU, ABT, CD, and YL contributed to data analysis. JSA created novel information technology for trial operations. RJU and ABT drafted the manuscript. All authors provided critical revisions and approved the final manuscript.
inancial Disclosures The authors have no relevant financial disclosures. Abbreviations: HCQ, hydroxychloroquine; IQR, interquartile range; SD, standard deviation; RT-PCR, reverse transcriptase polymerase chain reaction; AST, aspartate aminotransferase; ALT alanine aminotransferase; U, units; WBC, white blood cell count; LDH, lactic acid dehydrogenase
References
Abarientos, Sperber, Shapiro, Aronow, Chao et al., Hydroxychloroquine in systemic lupus erythematosus and rheumatoid arthritis and its safety in pregnancy, Expert Opin Drug Saf
Arshad, Kilgore, Chaudhry, Treatment with Hydroxychloroquine, Azithromycin, and Combination in Patients Hospitalized with COVID-19, Int J Infect Dis
Bhimraj, Morgan, Shumaker, Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19, Clin Infect Dis
Cavalcanti, Zampieri, Rosa, Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19, N Engl J Med
Chen, Hu, Zhang, Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial, medRxiv
Dong, Dh, Gardner, An interactive web-based dashboard to track COVID-19 in real time
Fanouriakis, Kostopoulou, Alunno, update of the EULAR recommendations for the management of systemic lupus erythematosus, Ann Rheum Dis
Furst, Lindsley, Baethge, Dose-loading with hydroxychloroquine improves the rate of response in early, active rheumatoid arthritis: a randomized, double-blind six-week trial with eighteen-week extension, Arthritis Rheum
Gautret, Lagier, Parola, Hydroxychloroquine and Azithromycin as a treatment of COVID-19: preliminary results of an open-label non-randomized clinical trial, medRxiv
Geleris, Sun, Platt, Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19, N Engl J Med
Hernandez, Roman, Pasupuleti, Barboza, White, Hydroxychloroquine or Chloroquine for Treatment or Prophylaxis of COVID-19: A Living Systematic Review, Ann Intern Med
Horby, Mafham, Linsell, Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19: Preliminary results from a multi-centre, randomized, controlled trial, medRxiv
Hunt, Johnson, Calcium requirements: new estimations for men and women by cross-sectional statistical analyses of calcium balance data from metabolic studies, Am J Clin Nutr
Lagier, Million, Gautret, Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis, Travel Med Infect Dis
Liu, Cao, Xu, Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro, Cell Discov
Lopes, The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review, Clin Kidney J
Maa, Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases, Pharmacol Res Perspect
Mercuro, Yen, Shim, Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease
Petkova, Antman, Troxel, Pooling Data From Individual Clinical Trials in the COVID-19 Era, JAMA
Rosenberg, Dufort, Udo, Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State, JAMA
Tang, Cao, Han, Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial, BMJ
Van Den Borne, Be, Dijkmans, De Rooij, Le Cessie et al., Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells, J Rheumatol
Vannice, Cassetti, Eisinger, Demonstrating vaccine effectiveness during a waning epidemic: A WHO/NIH meeting report on approaches to development and licensure of Zika vaccine candidates, Vaccine
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Wang, Hu, Hu, Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China, JAMA
Whyte, Kelly, Gonzalez, Arya, Roberts, Pulmonary embolism in hospitalised patients with COVID-19, Thromb Res
Wilson, COVID-19: Interim Guidance on Management Pending Empirical Evidence
Wu, Chang, Hsu, Hydroxychloroquine inhibits CD154 expression in CD4(+) T lymphocytes of systemic lupus erythematosus through NFAT, but not STAT5, signaling, Arthritis Res Ther
Yachida, Kuwahara, Iritani, Hayashi, In ovo interference of embryo nonlethal avian infectious bronchitis viruses (IBV) with velogenic Newcastle disease virus and embryo adapted IBV, Res Vet Sci
Yao, Ye, Zhang, Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Clin Infect Dis
Yu, Li, Chen, Evaluation of variation in D-dimer levels among COVID-19 and bacterial pneumonia: a retrospective analysis, J Thromb Thrombolysis
Zeng, Huang, Guo, Association of inflammatory markers with the severity of COVID-19: A meta-analysis, Int J Infect Dis
DOI record:
{
"DOI": "10.1093/ofid/ofaa446",
"ISSN": [
"2328-8957"
],
"URL": "http://dx.doi.org/10.1093/ofid/ofaa446",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Effective therapies to combat coronavirus 2019 (COVID-19) are urgently needed. Hydroxychloroquine (HCQ) has in vitro antiviral activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), but the clinical benefit of HCQ in treating COVID-19 is unclear. Randomized controlled trials are needed to determine the safety and efficacy of HCQ for the treatment of hospitalized patients with COVID-19.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>We conducted a multicenter, double-blind randomized clinical trial of HCQ among patients hospitalized with laboratory-confirmed COVID-19. Subjects were randomized in a 1:1 ratio to HCQ or placebo for 5 days and followed for 30 days. The primary efficacy outcome was a severe disease progression composite end point (death, intensive care unit admission, mechanical ventilation, extracorporeal membrane oxygenation, and/or vasopressor use) at day 14.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>A total of 128 patients were included in the intention-to-treat analysis. Baseline demographic, clinical, and laboratory characteristics were similar between the HCQ (n = 67) and placebo (n = 61) arms. At day 14, 11 (16.4%) subjects assigned to HCQ and 6 (9.8%) subjects assigned to placebo met the severe disease progression end point, but this did not achieve statistical significance (P = .350). There were no significant differences in COVID-19 clinical scores, number of oxygen-free days, SARS-CoV-2 clearance, or adverse events between HCQ and placebo. HCQ was associated with a slight increase in mean corrected QT interval, an increased D-dimer, and a trend toward an increased length of stay.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>In hospitalized patients with COVID-19, our data suggest that HCQ does not prevent severe outcomes or improve clinical scores. However, our conclusions are limited by a relatively small sample size, and larger randomized controlled trials or pooled analyses are needed.</jats:p>\n </jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-3217-5062",
"affiliation": [
{
"name": "Department of Medicine, New York University Grossman School of Medicine, New York, New York, USA"
},
{
"name": "Division of Infectious Diseases and Immunology, New York University Grossman School of Medicine, New York, New York, USA"
}
],
"authenticated-orcid": false,
"family": "Ulrich",
"given": "Robert J",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Population Health, New York University Grossman School of Medicine, New York, New York, USA"
},
{
"name": "Division of Biostatistics, New York University Grossman School of Medicine, New York, New York, USA"
}
],
"family": "Troxel",
"given": "Andrea B",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, New York University Grossman School of Medicine, New York, New York, USA"
},
{
"name": "Division of Infectious Diseases and Immunology, New York University Grossman School of Medicine, New York, New York, USA"
}
],
"family": "Carmody",
"given": "Ellie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, New York University Grossman School of Medicine, New York, New York, USA"
},
{
"name": "Division of Infectious Diseases and Immunology, New York University Grossman School of Medicine, New York, New York, USA"
}
],
"family": "Eapen",
"given": "Jaishvi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Division of Infectious Diseases, NYU Long Island School of Medicine, Mineola, New York, USA"
}
],
"family": "Bäcker",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Division of Infectious Diseases, State University of New York Downstate Health Sciences University, Brooklyn, New York, USA"
}
],
"family": "DeHovitz",
"given": "Jack A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, New York University Grossman School of Medicine, New York, New York, USA"
},
{
"name": "Division of Infectious Diseases and Immunology, New York University Grossman School of Medicine, New York, New York, USA"
}
],
"family": "Prasad",
"given": "Prithiv J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Population Health, New York University Grossman School of Medicine, New York, New York, USA"
},
{
"name": "Division of Biostatistics, New York University Grossman School of Medicine, New York, New York, USA"
}
],
"family": "Li",
"given": "Yi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "New York University Grossman School of Medicine, New York, New York, USA"
}
],
"family": "Delgado",
"given": "Camila",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, New York University Grossman School of Medicine, New York, New York, USA"
}
],
"family": "Jrada",
"given": "Morris",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pediatrics, New York University Grossman School of Medicine, New York, New York, USA"
},
{
"name": "Division of Pediatric Hematology-Oncology, New York University Grossman School of Medicine, New York, New York, USA"
}
],
"family": "Robbins",
"given": "Gabriel A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, New York University Grossman School of Medicine, New York, New York, USA"
},
{
"name": "Division of Infectious Diseases and Immunology, New York University Grossman School of Medicine, New York, New York, USA"
}
],
"family": "Henderson",
"given": "Brooklyn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, New York University Grossman School of Medicine, New York, New York, USA"
},
{
"name": "Division of Infectious Diseases and Immunology, New York University Grossman School of Medicine, New York, New York, USA"
}
],
"family": "Hrycko",
"given": "Alexander",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Division of Infectious Diseases, NYU Long Island School of Medicine, Mineola, New York, USA"
}
],
"family": "Delpachitra",
"given": "Dinuli",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2579-1104",
"affiliation": [
{
"name": "Department of Medicine, New York University Grossman School of Medicine, New York, New York, USA"
},
{
"name": "Department of Pediatrics, New York University Grossman School of Medicine, New York, New York, USA"
},
{
"name": "Division of Infectious Diseases and Immunology, New York University Grossman School of Medicine, New York, New York, USA"
},
{
"name": "Divison of Pediatric Infectious Diseases, New York University Grossman School of Medicine, New York, New York, USA"
}
],
"authenticated-orcid": false,
"family": "Raabe",
"given": "Vanessa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, New York University Grossman School of Medicine, New York, New York, USA"
}
],
"family": "Austrian",
"given": "Jonathan S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, New York University Grossman School of Medicine, New York, New York, USA"
},
{
"name": "Division of Infectious Diseases and Immunology, New York University Grossman School of Medicine, New York, New York, USA"
},
{
"name": "Department of Pharmacy, NYU Langone Health, New York, New York, USA"
}
],
"family": "Dubrovskaya",
"given": "Yanina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, New York University Grossman School of Medicine, New York, New York, USA"
},
{
"name": "Division of Infectious Diseases and Immunology, New York University Grossman School of Medicine, New York, New York, USA"
}
],
"family": "Mulligan",
"given": "Mark J",
"sequence": "additional"
}
],
"container-title": "Open Forum Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
9,
23
]
],
"date-time": "2020-09-23T05:34:34Z",
"timestamp": 1600839274000
},
"deposited": {
"date-parts": [
[
2020,
10,
27
]
],
"date-time": "2020-10-27T15:06:39Z",
"timestamp": 1603811199000
},
"funder": [
{
"name": "New York University Grossman School of Medicine"
},
{
"award": [
"TL1 TR001445",
"UL1 TR001445"
],
"name": "NYU CTSA"
},
{
"DOI": "10.13039/100006108",
"doi-asserted-by": "publisher",
"name": "National Center for Advancing Translational Sciences"
},
{
"name": "New York State Empire Clinical Research Investigator Program"
},
{
"DOI": "10.13039/100000060",
"doi-asserted-by": "publisher",
"name": "National Institute of Allergy and Infectious Diseases"
},
{
"DOI": "10.13039/100000002",
"award": [
"UM1 AI148574"
],
"doi-asserted-by": "publisher",
"name": "National Institutes of Health"
},
{
"award": [
"D43 TW010046",
"D43 TW010562",
"D43 TW011532"
],
"name": "National Institutes of Health Fogarty"
}
],
"indexed": {
"date-parts": [
[
2024,
3,
13
]
],
"date-time": "2024-03-13T06:50:09Z",
"timestamp": 1710312609724
},
"is-referenced-by-count": 47,
"issue": "10",
"issued": {
"date-parts": [
[
2020,
9,
23
]
]
},
"journal-issue": {
"issue": "10",
"published-print": {
"date-parts": [
[
2020,
10,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
9,
23
]
],
"date-time": "2020-09-23T00:00:00Z",
"timestamp": 1600819200000
}
}
],
"link": [
{
"URL": "http://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofaa446/33782144/ofaa446.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "http://academic.oup.com/ofid/article-pdf/7/10/ofaa446/34043569/ofaa446.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "http://academic.oup.com/ofid/article-pdf/7/10/ofaa446/34043569/ofaa446.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2020,
9,
23
]
]
},
"published-online": {
"date-parts": [
[
2020,
9,
23
]
]
},
"published-other": {
"date-parts": [
[
2020,
10
]
]
},
"published-print": {
"date-parts": [
[
2020,
10,
1
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"author": "Dong",
"key": "2020102711010509200_CIT0001"
},
{
"DOI": "10.1038/s41421-020-0156-0",
"article-title": "Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "16",
"journal-title": "Cell Discov",
"key": "2020102711010509200_CIT0002",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "Cell Res",
"key": "2020102711010509200_CIT0003",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1002/prp2.293",
"article-title": "Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases",
"author": "Al-Bari",
"doi-asserted-by": "crossref",
"first-page": "e00293",
"journal-title": "Pharmacol Res Perspect",
"key": "2020102711010509200_CIT0004",
"volume": "5",
"year": "2017"
},
{
"DOI": "10.1186/s13075-017-1393-y",
"article-title": "Hydroxychloroquine inhibits CD154 expression in CD4+ T lymphocytes of systemic lupus erythematosus through NFAT, but not STAT5, signaling",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "183",
"journal-title": "Arthritis Res Ther",
"key": "2020102711010509200_CIT0005",
"volume": "19",
"year": "2017"
},
{
"article-title": "Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells",
"author": "van den Borne",
"first-page": "55",
"journal-title": "J Rheumatol",
"key": "2020102711010509200_CIT0006",
"volume": "24",
"year": "1997"
},
{
"DOI": "10.1517/14740338.2011.566555",
"article-title": "Hydroxychloroquine in systemic lupus erythematosus and rheumatoid arthritis and its safety in pregnancy",
"author": "Abarientos",
"doi-asserted-by": "crossref",
"first-page": "705",
"journal-title": "Expert Opin Drug Saf",
"key": "2020102711010509200_CIT0007",
"volume": "10",
"year": "2011"
},
{
"DOI": "10.1136/annrheumdis-2019-215089",
"article-title": "2019 update of the EULAR recommendations for the management of systemic lupus erythematosus",
"author": "Fanouriakis",
"doi-asserted-by": "crossref",
"first-page": "736",
"journal-title": "Ann Rheum Dis",
"key": "2020102711010509200_CIT0008",
"volume": "78",
"year": "2019"
},
{
"author": "China National Health Commission.",
"edition": "7",
"key": "2020102711010509200_CIT0009",
"volume-title": "Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment.",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.06.099",
"article-title": "Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19",
"author": "Arshad",
"doi-asserted-by": "crossref",
"first-page": "396",
"journal-title": "Int J Infect Dis",
"key": "2020102711010509200_CIT0010",
"volume": "97",
"year": "2020"
},
{
"author": "Wilson",
"key": "2020102711010509200_CIT0011"
},
{
"author": "US Food and Drug Administration.",
"key": "2020102711010509200_CIT0012"
},
{
"DOI": "10.1101/2020.03.16.20037135",
"article-title": "Hydroxychloroquine and azithromycin as a treatment of COVID-19: preliminary results of an open-label non-randomized clinical trial",
"author": "Gautret",
"doi-asserted-by": "crossref",
"key": "2020102711010509200_CIT0013"
},
{
"article-title": "Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial",
"author": "Chen",
"journal-title": "medRxiv",
"key": "2020102711010509200_CIT0014",
"year": "2020"
},
{
"DOI": "10.1016/j.tmaid.2020.101791",
"article-title": "Outcomes of 3737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: a retrospective analysis",
"author": "Lagier",
"doi-asserted-by": "crossref",
"first-page": "101791",
"journal-title": "Travel Med Infect Dis",
"key": "2020102711010509200_CIT0015",
"volume": "36",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2012410",
"article-title": "Observational study of hydroxychloroquine in hospitalized patients with Covid-19",
"author": "Geleris",
"doi-asserted-by": "crossref",
"first-page": "2411",
"journal-title": "N Engl J Med",
"key": "2020102711010509200_CIT0016",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.8630",
"article-title": "Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state",
"author": "Rosenberg",
"doi-asserted-by": "crossref",
"first-page": "2493",
"journal-title": "JAMA",
"key": "2020102711010509200_CIT0017",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m1849",
"article-title": "Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial",
"author": "Tang",
"doi-asserted-by": "crossref",
"first-page": "m1849",
"journal-title": "BMJ",
"key": "2020102711010509200_CIT0018",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1001/jamacardio.2020.1834",
"article-title": "Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19)",
"author": "Mercuro",
"doi-asserted-by": "crossref",
"first-page": "1036",
"journal-title": "JAMA Cardiol",
"key": "2020102711010509200_CIT0019",
"volume": "5",
"year": "2020"
},
{
"article-title": "Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19",
"author": "Bhimraj",
"journal-title": "Clin Infect Dis",
"key": "2020102711010509200_CIT0020",
"year": "2020"
},
{
"author": "US Food and Drug Administration",
"key": "2020102711010509200_CIT0021"
},
{
"DOI": "10.7326/M20-2496",
"article-title": "Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review",
"author": "Hernandez",
"doi-asserted-by": "crossref",
"first-page": "287",
"journal-title": "Ann Intern Med",
"key": "2020102711010509200_CIT0022",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.1002/1529-0131(199902)42:2<357::AID-ANR19>3.0.CO;2-J",
"article-title": "Dose-loading with hydroxychloroquine improves the rate of response in early, active rheumatoid arthritis: a randomized, double-blind six-week trial with eighteen-week extension",
"author": "Furst",
"doi-asserted-by": "crossref",
"first-page": "357",
"journal-title": "Arthritis Rheum",
"key": "2020102711010509200_CIT0023",
"volume": "42",
"year": "1999"
},
{
"DOI": "10.1093/cid/ciaa237",
"article-title": "In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)",
"author": "Yao",
"doi-asserted-by": "crossref",
"first-page": "732",
"journal-title": "Clin Infect Dis",
"key": "2020102711010509200_CIT0024",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1093/ckj/sfs160",
"article-title": "The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review",
"author": "Lopes",
"doi-asserted-by": "crossref",
"first-page": "8",
"journal-title": "Clin Kidney J",
"key": "2020102711010509200_CIT0025",
"volume": "6",
"year": "2013"
},
{
"author": "National Institue of Health",
"key": "2020102711010509200_CIT0026"
},
{
"DOI": "10.1016/j.ijid.2020.05.055",
"article-title": "Association of inflammatory markers with the severity of COVID-19: a meta-analysis",
"author": "Zeng",
"doi-asserted-by": "crossref",
"first-page": "467",
"journal-title": "Int J Infect Dis",
"key": "2020102711010509200_CIT0027",
"volume": "96",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.1585",
"article-title": "Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1061",
"journal-title": "JAMA",
"key": "2020102711010509200_CIT0028",
"volume": "323",
"year": "2020"
},
{
"article-title": "Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial",
"author": "Horby",
"journal-title": "medRxiv",
"key": "2020102711010509200_CIT0029",
"year": "2020"
},
{
"key": "2020102711010509200_CIT0030"
},
{
"article-title": "Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19",
"author": "Cavalcanti",
"journal-title": "N Engl J Med",
"key": "2020102711010509200_CIT0031",
"year": "."
},
{
"key": "2020102711010509200_CIT0032"
},
{
"DOI": "10.1007/s11239-020-02171-y",
"article-title": "Evaluation of variation in D-dimer levels among COVID-19 and bacterial pneumonia: a retrospective analysis",
"author": "Yu",
"doi-asserted-by": "crossref",
"first-page": "548",
"journal-title": "J Thromb Thrombolysis",
"key": "2020102711010509200_CIT0033",
"volume": "50",
"year": "2020"
},
{
"DOI": "10.1016/j.thromres.2020.07.025",
"article-title": "Pulmonary embolism in hospitalised patients with COVID-19",
"author": "Whyte",
"doi-asserted-by": "crossref",
"first-page": "95",
"journal-title": "Thromb Res",
"key": "2020102711010509200_CIT0034",
"volume": "195",
"year": "2020"
},
{
"key": "2020102711010509200_CIT0035"
},
{
"DOI": "10.1016/S0034-5288(18)30477-6",
"article-title": "In ovo interference of embryo non-lethal avian infectious bronchitis viruses (IBV) with velogenic Newcastle disease virus and embryo adapted IBV",
"author": "Yachida",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Res Vet Sci",
"key": "2020102711010509200_CIT0036",
"volume": "40",
"year": "1986"
},
{
"DOI": "10.1016/j.vaccine.2018.12.040",
"article-title": "Demonstrating vaccine effectiveness during a waning epidemic: a WHO/NIH meeting report on approaches to development and licensure of Zika vaccine candidates",
"author": "Vannice",
"doi-asserted-by": "crossref",
"first-page": "863",
"journal-title": "Vaccine",
"key": "2020102711010509200_CIT0037",
"volume": "37",
"year": "2019"
},
{
"author": "COVID-19 Collaborative Platform–COVID-CP",
"key": "2020102711010509200_CIT0038"
},
{
"DOI": "10.1001/jama.2020.13042",
"article-title": "Pooling data from individual clinical trials in the COVID-19 era",
"author": "Petkova",
"doi-asserted-by": "crossref",
"first-page": "543",
"journal-title": "JAMA",
"key": "2020102711010509200_CIT0039",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1093/ajcn/86.4.1054",
"article-title": "Calcium requirements: new estimations for men and women by cross-sectional statistical analyses of calcium balance data from metabolic studies",
"author": "Hunt",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "Am J Clin Nutr",
"key": "2020102711010509200_CIT0040",
"volume": "86",
"year": "2007"
}
],
"reference-count": 40,
"references-count": 40,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ofid/article/doi/10.1093/ofid/ofaa446/5910201"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Oncology"
],
"subtitle": [],
"title": "Treating COVID-19 With Hydroxychloroquine (TEACH): A Multicenter, Double-Blind Randomized Controlled Trial in Hospitalized Patients",
"type": "journal-article",
"volume": "7"
}
